Veterinary dermatology has undergone a radical transformation over the past two decades, marked by the emergence and global spread of methicillin-resistant staphylococci (MRS). Once considered simple bacterial infections that responded predictably to treatments, canine pyoderma is now at the center of an antimicrobial resistance crisis.
September 2025
Introduction: The Emergence of a Clinical and Public Health Challenge
This evolution has forced the veterinary profession to fundamentally reconsider its diagnostic, therapeutic, and biosecurity approaches. This report aims to provide a comprehensive analysis of the importance of MRS in veterinary dermatology, drawing on current scientific data to inform clinical practice and public health.
The main challenge lies in the fact that methicillin resistance, which confers clinical ineffectiveness to the entire class of beta-lactams, is very frequently associated with a multidrug resistance (MDR) phenotype. This association renders numerous other antibiotic families ineffective, such as macrolides, lincosamides, fluoroquinolones, or aminoglycosides. This situation has generated a therapeutic paradigm shift. The failure of empirical treatments, once effective, forces clinicians to resort to last-resort antibiotic molecules, such as chloramphenicol or rifampin. These alternatives, while potentially effective if the antibiogram confirms it, present more restrictive adverse effect profiles and require closer biological monitoring, thus increasing the complexity, risks, and cost of management.
Note that for GEDAC (French Study Group in Companion Animal Dermatology), rifampin is among the antibiotics that should never be used in order to preserve it for human medicine to treat serious human infections such as tuberculosis. The same applies to mupirocin.
Historical context and paradigm shift
The post-World War II era introduced antimicrobials to veterinary medicine, revolutionizing the treatment of infectious diseases. For decades, canine pyoderma, primarily caused by staphylococci, was successfully treated with β-lactam antibiotics, such as penicillins and cephalosporins. However, this widespread, often empirical use exerted intense selection pressure on bacterial populations. The inevitable result of this pressure was the emergence of resistant strains, transforming a manageable clinical problem into a major therapeutic challenge. The significant emergence of methicillin-resistant Staphylococcus pseudintermedius (MRSP) around the mid-2000s marked a turning point, signaling the end of the era of confidence in empirical treatments for pyoderma. This is not an isolated event, but the direct and predictable consequence of our past therapeutic practices. As canine pyoderma is an extremely common condition, and the first-line treatment was historically a β-lactam antibiotic, repeated use of these medications created an ideal evolutionary environment for the selection and spread of strains carrying resistance genes. Thus, our own treatment protocols have unwittingly shaped the emergence of the pathogens that are now the most difficult to treat.
Definition and taxonomy
Methicillin resistance in staphylococci is a phenotype conferring resistance to virtually all antibiotics in the β-lactam class, including penicillins, cephalosporins, and carbapenems. The underlying molecular mechanism is the presence of the mecA gene, which is located on a mobile genetic element called the staphylococcal chromosomal cassette (SCCmec). This gene codes for a modified penicillin-binding protein, PBP2a (Penicillin-Binding Protein 2a), which has very low affinity for β-lactam antibiotics. Consequently, bacterial cell wall synthesis can continue even in the presence of these antibiotics, rendering them ineffective.
The multidrug resistance (MDR) phenotype frequently observed in MRS is not a fortuitous phenomenon. The SCCmec cassette is a genetic platform that, in addition to the mecA gene, can carry and transfer genes conferring resistance to other classes of antibiotics (for example, tetracyclines, macrolides). Thus, the selection pressure exerted by the use of a beta-lactam can not only select strains resistant to methicillin, but also co-select, in a single event, resistance to multiple other antibiotic families. This phenomenon of co-selection explains the rapid emergence of quasi-untreatable strains and reinforces the argument against the empirical use of beta-lactams in staphylococcal infections.
A fundamental point for understanding current epidemiology is the taxonomic reclassification that occurred in 2005. Previously, most coagulase-positive staphylococci isolated from dogs were identified as Staphylococcus intermedius. Molecular techniques allowed the distinction of three distinct species within the Staphylococcus intermedius group (SIG): S. intermedius, S. delphini, and S. pseudintermedius. This revision revealed that S. pseudintermedius is the predominant species that colonizes and causes infections in dogs and cats. Consequently, the vast majority of isolates previously identified as S. intermedius in veterinary literature were actually S. pseudintermedius. In canine dermatology, three species of MRS are particularly relevant: S. pseudintermedius (MRSP), S. aureus (MRSA), and S. schleiferi (MRSS).
Global importance and the “One Health” concept
The problem of MRS in companion animals extends far beyond the walls of the veterinary clinic to become a public health concern. This situation fits perfectly within the framework of the “One Health” concept, which recognizes the deep and inseparable interconnection between human health, animal health, and environmental health. Companion animals share our domestic environment, often receive similar classes of antibiotics to those used in human medicine, and can serve as reservoirs or vectors for resistant bacteria. The presence of MRSA and MRSP in companion animals places them at the center of a dynamic transmission “triangle” between humans, animals, and their shared environment. The management of antibiotic resistance in veterinary dermatology is therefore not only a matter of animal welfare, but also a shared responsibility in the global fight against the growing threat of “superbugs”.
Epidemiology and Evolution of Resistant Staphylococci
The epidemiology of MRS in veterinary medicine is a dynamic field, characterized by the rapid emergence of resistant clones and their global dissemination. Understanding the prevalence, risk factors, and reservoirs of these pathogens is essential for effective clinical and preventive management.
A. Methicillin-Resistant Staphylococcus pseudintermedius (MRSP): The Dominant Canine Pathogen
History of emergence
Although sporadic cases were reported in the 1990s, the significant emergence of MRSP as a major clinical pathogen is a relatively recent phenomenon, which gained momentum from 2006. The first official recognitions date from 2001 in North America and 2007 in Europe. This rapid emergence was not the result of multiple and independent resistance events, but was mainly due to the explosive clonal spread of a few highly successful bacterial lineages.
Global clonal spread
Analysis by multilocus sequence typing (MLST) has allowed mapping of the global dissemination of MRSP. A systematic review identified five major clonal complexes (CC) that dominate the epidemiological landscape.
- CC71: Initially described as the “European epidemic clone”, it is now widespread worldwide, although the majority of isolates still come from Europe.
- CC68: Known as the “North American epidemic clone”, it is frequently isolated in North America but has also been identified in Europe.
- CC258: Increasingly reported in Europe, this clone presents a resistance profile distinct from CC71.
- CC45 and CC112: These lineages are particularly prevalent in Asia, but have also been detected on other continents.
This dynamic illustrates a two-step process: first, local selection of these clones by pressure exerted by antibiotic use in veterinary medicine, particularly beta-lactams; second, their global dissemination facilitated by increasing international movements of companion animals. This rapid dissemination over vast geographic areas is greatly facilitated by international movements of dogs, whether for shows, breeding, or travel. A study conducted in Grenada documented for the first time the introduction of MRSP to the island, with genotypic and epidemiological data suggesting that “visiting” dogs transmitted the pathogen to the local canine population.
Prevalence
The prevalence of MRSP varies dramatically depending on the canine population studied, which has direct implications for clinical suspicion.
- In healthy dogs: MRSP carriage in healthy dogs is generally low. Studies report prevalence rates ranging from 0% to 4.5%. These animals are asymptomatic carriers.
- In sick dogs: The situation is radically different in populations of dogs presented for dermatological problems. In specialized dermatology clinics, the prevalence of MRSP in dogs with pyoderma can be extremely high, reaching up to 40.5% in one study. Another study conducted in Thailand on dogs with pyoderma revealed an overall prevalence of MRS of 35.7%. This disparity underscores that dogs with chronic skin diseases constitute a very high-risk population. A large-scale study conducted in the United States between 2019 and 2022 analyzed tens of thousands of clinical isolates and identified 110,423 isolates of MRSP. In France, more recent data from sick animals report a frequency of MRSP of 17%.
Risk factors
Identification of risk factors is crucial for targeting diagnostic and prevention efforts. Several factors have been consistently associated with an increased risk of infection or colonization by MRSP:
- Previous antibiotic use: This is the most important and frequently cited risk factor. Recent exposure to antibiotics, particularly β-lactams and fluoroquinolones, selects resistant strains by eliminating competing sensitive flora.
- Contact with the veterinary environment: Recent hospitalizations or frequent visits to veterinary clinics increase the risk of exposure to MRSP.
- Underlying chronic skin diseases: Dogs suffering from atopic dermatitis, allergies, or chronic otitis are particularly predisposed. These conditions alter the skin barrier and often require repeated antimicrobial treatments, creating a vicious cycle.
Environmental reservoirs
Staphylococci are robust bacteria capable of surviving for long periods on inanimate surfaces. Veterinary clinics act as important environmental reservoirs for MRSP. One study showed that MRSP could be isolated on 10% of surfaces sampled in primary care clinics, with no significant difference between hand-contact surfaces (e.g., door handles, keyboards) and animal-contact surfaces (e.g., examination tables). Strain typing revealed great similarity between isolates from the same hospital, but low similarity between hospitals, indicating intra-hospital transmission and persistence. Indistinguishable strains of MRSP were found both in canine patients and on environmental surfaces within the same clinic, confirming the role of the environment in nosocomial transmission. Inadequate hand hygiene by veterinary staff, with compliance reported as low as 14%, is a major factor contributing to this transmission.
B. Methicillin-Resistant Staphylococcus aureus (MRSA) and S. schleiferi (MRSS): A Comparative Perspective
While MRSP is the main player, other MRS play a role in canine dermatology.
Staphylococcus aureus (MRSA)
- S. aureus is primarily a human commensal and pathogen. Its presence in dogs is therefore epidemiologically distinct from that of S. pseudintermedius.
- Origin and Transmission: S. aureus is not a member of the normal commensal flora of dogs. Consequently, when MRSA is isolated from a dog, it is most often a transient colonization or infection resulting from human-to-animal transmission, a phenomenon known as reverse zoonosis or anthroponosis. This colonization is generally short-lived, with the organism being eliminated within a few weeks if there is no re-exposure.
- Prevalence and Risk Factors: The prevalence of MRSA carriage in healthy dogs is low, generally between 0 and 4%. However, this rate may be higher in specific populations of animals having close contact with the human health system, such as therapy dogs visiting hospitals or pets belonging to healthcare professionals. Risk factors identified in companion animals for MRSA acquisition reflect those in humans: prior antibiotic therapy, hospitalization, and contact with a person infected or colonized with MRSA.
- Clinical Importance: Although less frequent than MRSP, MRSA is an opportunistic pathogen capable of causing clinical infections in dogs, particularly pyoderma, surgical site infections, and urinary tract infections.
Staphylococcus schleiferi (MRSS)
- S. schleiferi, which exists in two subspecies (S. schleiferi subsp. schleiferi, coagulase-negative, and S. schleiferi subsp. coagulans, coagulase-positive), is increasingly recognized as an important skin pathogen in dogs.
- Emerging Pathogen: It is now identified as a significant cause of pyoderma and canine external otitis. In some studies, it is the second most frequently isolated staphylococcus in these conditions.
- High Resistance Rates: A concerning characteristic of S. schleiferi is its high rate of methicillin resistance. Studies conducted in dermatology reference centers have reported resistance rates of 40% to 46.6%. A study on a less biased population found a resistance rate of 20% in dogs with inflammatory dermatitis.
- Multidrug Resistance: Like MRSP, MRSS often exhibits multidrug resistance, particularly frequent resistance to fluoroquinolones, further complicating therapeutic options.
The distinction between these three pathogens is of paramount importance in clinical practice. A laboratory report identifying a “Methicillin-resistant Staphylococcus” requires informed interpretation to guide case management, client communication, and biosecurity measures. For example, identification of MRSA (S. aureus) should immediately raise suspicion of a human source and trigger discussion about potential risks to household members’ health. Conversely, a diagnosis of MRSP will direct investigation toward managing the dog’s underlying skin disease and controlling environmental contamination. The following table summarizes these key differences.
|
Characteristic |
Staphylococcus pseudintermedius (MRSP) |
Staphylococcus aureus (MRSA) |
Staphylococcus schleiferi (MRSS) |
|
Primary Commensal Status |
Dog (normal flora of skin and mucous membranes) |
Human (transient canine colonization) |
Dog and Human (skin flora) |
|
Prevalence in Canine Pyoderma |
Very high (dominant pathogen) |
Low to moderate |
Low to moderate (emerging pathogen) |
|
Primary Reservoir |
Animal (dog) |
Human (healthcare system, community) |
Animal and Human |
|
Primary Transmission Route |
Animal-Animal, Environment-Animal |
Human-Animal (reverse zoonosis) |
Less defined, probably Animal-Animal |
|
Zoonotic Potential (Animal → Human) |
Low but documented |
Significant (major public health concern) |
Rare, mainly nosocomial in humans |
Pathogenesis of Staphylococcal Pyoderma
Staphylococcal pyoderma is not simply the presence of bacteria on the skin; it is the result of a complex interaction between the pathogen, the host, and the environment. The transition of S. pseudintermedius from a simple skin resident to an infectious agent depends on the disruption of the delicate balance that maintains skin homeostasis.
From Commensal to Opportunistic Pathogen: The Role of the Skin Barrier
- S. pseudintermedius is a commensal well adapted to its canine host, colonizing the skin and mucous membranes (nostrils, mouth, pharynx, perineum) of 46% to 92% of healthy dogs without causing disease. Infection is an opportunistic event, which only occurs when host defenses are weakened. The skin barrier, composed of physical (stratum corneum), chemical (lipids, antimicrobial peptides), and immunological elements, is the primary line of defense. Any alteration of this barrier creates a gateway for bacterial invasion.
The clinical importance of MRSP does not lie in increased virulence compared to its sensitive counterpart (MSSP). Indeed, studies have not demonstrated that methicillin-resistant strains are intrinsically more aggressive or pathogenic. The disease mechanisms and clinical lesions they cause are indistinguishable. The real challenge lies elsewhere: it resides in therapeutic failure. Faced with an infection with MRSP, empirical treatment with a first-line antibiotic, such as cephalexin, is doomed to failure. This initial failure triggers a cascade of negative consequences: the disease is prolonged, increasing morbidity and discomfort for the animal; the bacterial load on the animal and in its environment increases, raising the risk of transmission; and this often leads to cycles of successive and ineffective antibiotic therapies, which not only fail to resolve the problem, but exert additional selection pressure, favoring the emergence of additional resistance. Thus, a simple pyoderma transforms into a complex, chronic, costly, and frustrating clinical case, with potential implications for public health. It is this dimension of therapeutic failure that gives MRSP its devastating clinical importance.
Role of Virulence Factors
Once the barrier is breached, S. pseudintermedius uses an arsenal of virulence factors to establish infection.
- Exfoliative Toxins (ExpA and ExpB): These proteins are key players in pyoderma lesion formation. They act as highly specific proteases that target and cleave canine desmoglein-1, a desmosomal cadherin essential for adhesion between keratinocytes in the epidermis. Degradation of desmoglein-1 disrupts epidermal cohesion, causing intra-epidermal cleavage (acantholysis) and subcorneal pustule formation. Intradermal injection of these purified toxins in dogs reproduces the clinical signs of pyoderma, such as erythema and crust formation, confirming their direct pathogenic role. Other toxins include the exfoliative toxin SIET, a bicomponent leukotoxin, Luk-I, which targets and destroys polymorphonuclear leukocytes, and superantigenic enterotoxins, such as SECCANINE, capable of causing polyclonal T lymphocyte activation.
- Adhesins and Surface Proteins: To initiate infection, the bacterium must first adhere to host cells. S. pseudintermedius expresses a series of surface proteins, called adhesins (Sps family), which specifically bind to components of the host’s extracellular matrix, such as fibrinogen, fibronectin, and cytokeratin-10. Proteins like SpsD and SpsL facilitate not only adhesion to corneocytes, but also keratinocyte invasion and biofilm formation.
- Biofilm Formation: Biofilm is a structured community of bacteria embedded in a self-produced polymeric matrix, which provides protection against host immune defenses and antibiotics, thus contributing to infection chronicity. Biofilm formation is particularly critical in chronic infections, otitis, and surgical site infections, especially on inert materials like implants or sutures. Biofilm effectively protects bacteria from the host’s immune response and antibiotic penetration, making infection eradication extremely difficult.
The Central Predisposing Factor: Skin Barrier Disruption
The vast majority of cases of canine staphylococcal pyoderma are secondary diseases. The assertion that “healthy dogs do not develop skin infections” is a fundamental principle in veterinary dermatology. Identification and management of the underlying cause are therefore not only important for treatment, but absolutely essential for preventing recurrences.
- Atopic Dermatitis and Allergies: This is the most frequent underlying cause. Allergic inflammation (whether due to environmental allergens, food, or flea bites) compromises the skin barrier in several ways. First, the intense pruritus it causes leads to self-trauma (scratching, licking, chewing) that physically damages the stratum corneum. Second, inflammation itself alters the composition and organization of intercellular lipids (ceramides) and decreases the production of natural antimicrobial peptides, making the skin more permeable and less able to defend against microbes. This inflamed and damaged skin environment is an ideal breeding ground for S. pseudintermedius proliferation.
- Other Predisposing Factors: Other conditions can also alter skin integrity and predispose to infections. These include endocrinopathies such as hypothyroidism and hypercorticism (Cushing’s syndrome), which affect skin renewal and local immunity; ectoparasites (Demodex, Sarcoptes) that cause direct damage and inflammation; and keratinization disorders (seborrhea) that modify the skin surface.
Clinical and Diagnostic Approach to Resistant Staphylococcal Pyoderma
When faced with a case of pyoderma, the clinician must adopt a systematic and evidence-based approach. The era of antimicrobial resistance demands greater diagnostic rigor to avoid therapeutic failures and promote judicious antibiotic use.
A. Clinical Presentation
Staphylococcal skin infections in dogs are classified according to the depth of tissue involvement.
- Superficial Pyoderma: The infection is confined to the epidermis and hair follicles. The most common form is superficial bacterial folliculitis. Typical clinical lesions are varied and include:
- Follicular Papules and Pustules: Small red elevations (papules) or pus-filled (pustules), centered on a hair.
- Epidermal Collarettes: These are very characteristic lesions. They present as circular areas of alopecia with a border of scales or crusts that extends centrifugally.
- Target Lesions: Areas of central erythema with a paler border and a peripheral erythematous ring.
- “Moth-eaten” Appearance: In short-haired dogs, folliculitis may manifest as multiple small areas of alopecia, giving the coat a “moth-eaten” appearance.
- Other signs such as crusts, erythema, and post-inflammatory hyperpigmentation are also frequent.
- Deep Pyoderma: This form is more severe as it involves hair follicle rupture (furunculosis) and extension of infection into the dermis and sometimes hypodermis (cellulitis). Clinical signs are more spectacular and often painful:
- Nodules and Hemorrhagic Bullae: Raised lesions, firm or fluctuant, often filled with purulent or bloody content.
- Draining Fistulas: Openings in the skin through which serosanguinous or purulent exudate flows.
- Severe Signs: Edema, ulcers, thick crusts, and marked inflammation are common. Unlike superficial pyoderma, dogs with deep pyoderma may present systemic signs such as fever, lethargy, and anorexia.
A point of paramount importance must be emphasized: it is clinically impossible to distinguish pyoderma caused by a methicillin-resistant staphylococcal strain from an infection caused by a sensitive strain. The lesions are morphologically identical. Suspicion of a resistant infection should therefore never be based on the appearance of lesions, but rather on the patient’s history, the presence of risk factors, and, especially, the response to treatment.
B. Evidence-Based Diagnostic Strategy
The advent of MRS has imposed a paradigm shift in the diagnostic approach to pyoderma. The historical approach of “treat first, ask questions later” with empirical antibiotics has not only become obsolete, but is now considered dangerous and contrary to the principles of antimicrobial stewardship. Cytology, once considered a complementary tool, has become an essential and non-negotiable step in the initial examination. Ignoring cytology in the current context of widespread resistance constitutes professional malpractice. It is the pivot of clinical decision: it confirms the presence of bacterial infection, thus justifying the initiation of antimicrobial treatment (which should be topical as first-line), it guides recognition of cases requiring immediate culture (presence of bacilli, deep pyoderma), and it allows evaluation of treatment response.
Skin Cytology: The Indispensable First-Line Tool
Cytology is a rapid, inexpensive, minimally invasive diagnostic test that can be performed in the clinic and should be systematically performed on any patient presenting lesions compatible with pyoderma.
- Sampling Technique: The method depends on the nature of the lesion.
- Direct impression on slide: For moist or exudative lesions, or after rupturing an intact pustule with a sterile needle.
- Tape test (Adhesive strip): Ideal for dry, scaly lesions or those located in hard-to-reach areas like interdigital spaces.
- Swab: Useful for collecting material from ear canals or fistulas.
- Interpretation: The sample is stained (for example, with a modified Wright-Giemsa stain) and examined under microscope. The discovery of cocci phagocytosed by degenerate neutrophils is pathognomonic proof of bacterial pyoderma. The presence of numerous extracellular bacteria associated with an inflammatory cell population is also highly suggestive. Cytology also allows detection of other agents (yeasts such as Malassezia, bacilli) and evaluation of the type of inflammatory response.
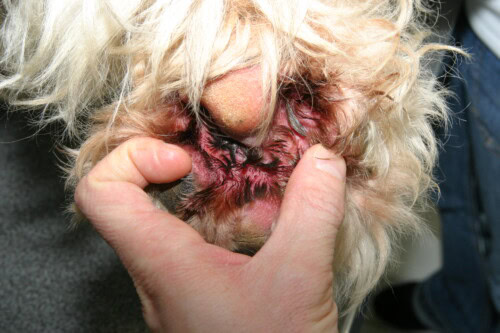
Cytology is the first essential examination to perform
Bacterial Culture and Antibiogram: The Key to Targeted Therapy
Bacterial culture with antimicrobial susceptibility testing (antibiogram) is not necessary for all cases of pyoderma, but it is imperative in specific circumstances to guide rational therapy.
- Clear Indications: Culture and antibiogram are strongly recommended, even mandatory, in the following situations:
- Insufficient clinical response (less than 50% improvement of lesions) after two weeks of appropriate systemic antibiotic therapy.
- Appearance of new lesions (papules, pustules) during ongoing antibiotic treatment for at least two weeks.
- Presence of bacilli on cytological examination.
- Presence of deep pyoderma lesions (furunculosis, cellulitis, nodules).
- History of multidrug-resistant infection in the animal or another animal in the household.
- Sampling Methodology: To be reliable, sampling for culture must be performed aseptically to avoid any contamination. Sample quality is determinant. Ideal sampling sites and techniques are:
- Intact pustule: This is the ideal site. After gentle disinfection, the pustule is ruptured sterily with a needle and its contents collected on a swab.
- Crust: The swab is passed over the moist surface of the skin after gently lifting a recent crust.
- Epidermal collarette: The swab is rolled firmly on the active border of the lesion.
- Nodule or fistula: Tissue biopsy for culture is the method of choice for deep lesions.
- Interpretation and Utility: The laboratory will identify the bacterial species and test its sensitivity to an antibiotic panel. For staphylococci, the sensitivity test to oxacillin or cefoxitin is used to detect methicillin resistance (presence of the mecA gene). The antibiogram is the only way to choose an effective systemic antibiotic against an MRSP strain, which is often multidrug-resistant.
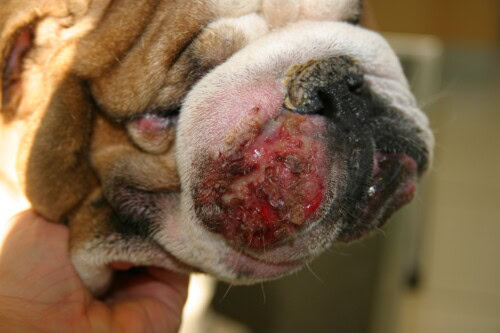
The antibiogram proves essential in MRS management
Therapeutic Principles and Management of MRSP Infections
The management of MRSP pyoderma has evolved toward a multimodal approach that emphasizes topical therapy, reserves systemic antibiotic therapy for specific indications, and, above all, addresses the underlying cause of infection.
A. Topical Therapy: Cornerstone of Treatment
Faced with rising resistance, topical therapy has been elevated from adjunct treatment status to first-line treatment. International guidelines, such as those from the World Association for Veterinary Dermatology (WAVD), now recommend topical therapy as the treatment modality of choice for superficial pyoderma, whether caused by sensitive or resistant strains. Several studies have demonstrated that a well-conducted topical treatment protocol can be as effective as systemic antibiotic therapy for resolving superficial pyoderma, including those caused by MRSP. The advantages are multiple: obtaining very high concentrations of antiseptics at the infection site (well above the minimum inhibitory concentration), reducing the risk of resistance selection in other systems (such as the digestive tract), and non-specific beneficial effects such as mechanical debridement of crusts and debris.
Effective Antiseptic Agents
Several active ingredients have proven their effectiveness.
- Chlorhexidine: This is the most studied and recommended topical agent due to its potent in vivo antimicrobial activity and residual effect. It is bactericidal against staphylococci, including methicillin-resistant strains. Effective concentrations in veterinary products generally range from 2% to 4%.
- Benzoyl Peroxide: At concentrations of 2.5% to 3%, it has antimicrobial activity, as well as keratolytic properties (helps remove dead skin layers), comedolytic (opens hair follicles), and degreasing. However, it can be drying and irritating to the skin, which may limit its use or require concomitant use of emollients.
- Other agents: Other antiseptics such as diluted sodium hypochlorite (diluted bleach), acetic acid/boric acid, and ethyl lactate have also shown some efficacy and may be valid options.
Application Protocols
The success of topical therapy depends critically on protocol compliance.
- Formulations: The choice of formulation depends on the extent of lesions, coat type, and ease of application for the owner.
- Shampoos: Ideal for generalized infections, they allow treatment of large skin surfaces.
- Foams, Sprays, Wipes: Very useful for daily application, for localized treatments, or for days without bathing. They generally do not require rinsing.
- Creams, Ointments, Gels: Suitable for localized lesions and areas with sparse hair.
- Frequency and Contact Time: A contact time of at least 10 minutes is crucial for antiseptic shampoos to exert their bactericidal effect. The recommended bathing frequency is generally 2 to 3 times per week, but can be increased to daily frequency in severe cases of MRSP. Foams and sprays can be applied once to twice daily. Treatment should be continued for at least 7 to 10 days after complete clinical resolution of lesions to prevent early relapses. For long or dense-coated animals, clipping is often indispensable to allow the product to reach the skin and act effectively.
Table 1: Topical Therapy Protocols for Canine Superficial Pyoderma
|
Active Ingredient |
Common Concentrations |
Available Formulations |
Recommended Application Protocol |
Clinical Remarks |
|
Chlorhexidine |
2%, 3%, 4% |
Shampoo, Foam, Spray, Wipes, Gel |
Shampoo: 2-3 times/week, 10-minute contact time. Foam/Spray/Wipes: 1-2 times/day, particularly on non-shampooing days. |
First-choice antiseptic. Very good efficacy and tolerance. Clipping significantly improves efficacy. |
|
Benzoyl peroxide |
2.5%, 3% |
Shampoo, Gel |
Shampoo: 2-3 times/week, 5-10 minute contact time. |
Keratolytic and degreasing action useful in certain cases. Higher irritating and drying potential than chlorhexidine. |
|
Sodium hypochlorite |
0.005% (dilution) |
Bath solution |
Baths: 2-3 times/week, 10-15 minute contact time. |
Interesting and inexpensive alternative. Extemporaneous preparation necessary (diluted bleach). |
|
Topical antibiotics (Mupirocin, Fusidic acid) |
2% |
Ointment, Cream |
Application: 2-3 times/day on very localized lesions. |
Reserved for focal lesions. Extensive use should be prohibited to limit the risk of resistance selection. Mupirocin should not be used (GEDAC Recommendations) |
B. Systemic Antibiotic Therapy: A Reasoned and Targeted Approach
The use of systemic antibiotics should be a thoughtful and justified decision, not a reflex. It is reserved for deep pyoderma, where infection reaches tissues that topical therapy cannot penetrate effectively, and for cases of generalized superficial pyoderma that do not respond to well-conducted topical treatment alone.
Selection Principle
The choice of a systemic antibiotic for a suspected or confirmed MRSP infection must imperatively be based on antibiogram results. Empirical use of second or third-line antibiotics is a dangerous practice that favors the emergence of even more extensive resistance. It is crucial to respect the “expert rule“: an oxacillin-resistant staphylococcus must be considered clinically resistant to all beta-lactams, even if the antibiogram suggests apparent sensitivity to certain cephalosporins. Treatment duration must be sufficiently long: at least 3 to 4 weeks for superficial pyoderma and 6 to 8 weeks for deep pyoderma, always continuing treatment for 1 to 2 weeks after complete clinical resolution.
Therapeutic Options for MRSP (based on sensitivity)
MRSP is, by definition, resistant to all β-lactam antibiotics. Moreover, epidemic clones are often multidrug-resistant, showing cross-resistance to many other antibiotic classes commonly used in veterinary medicine, such as tetracyclines, macrolides (erythromycin), lincosamides (clindamycin), and fluoroquinolones (enrofloxacin). Therapeutic options are therefore limited and must be chosen carefully based on culture results. The use of critically important antibiotics in human medicine (vancomycin, linezolid, teicoplanin) is strongly discouraged.
Table 2: Systemic Antibiotic Therapy Options for Resistant Staphylococcal Infections in Dogs (based on antibiogram)
|
Antibiotic |
Dosage (Dog) |
Administration Frequency |
Notable Side Effects |
Precautions and Recommended Monitoring |
|
Chloramphenicol |
50 mg/kg |
Every 8 hours (TID) |
Gastrointestinal disorders (vomiting, diarrhea), neurological weakness, reversible myelosuppression (dose-dependent). |
Narrow safety margin. Hematological monitoring recommended during prolonged treatments. Contraindicated in breeding animals. |
|
Doxycycline / Minocycline |
5-10 mg/kg |
Every 12 hours (BID) |
Gastrointestinal disorders (vomiting, esophagitis). Minocycline may be more effective than doxycycline on certain strains. |
Administer with food and water to reduce esophagitis risk. Photosensitivity is rare but possible. |
|
Clindamycin |
11 mg/kg |
Every 12 hours (BID) |
Gastrointestinal disorders. |
Often ineffective on MRSP due to frequent co-resistance. Efficacy conditioned by antibiogram. |
|
Rifampin |
5-10 mg/kg |
Every 24 hours (SID) |
Potentially severe hepatotoxicity, gastrointestinal disorders, orange coloration of urine and tears. |
Never use as monotherapy (risk of rapid selection of resistant mutants). Mandatory liver enzyme monitoring before and during treatment (e.g., at 2 and 4 weeks). Should never be used (GEDAC Recommendations) |
|
Potentiated sulfonamides (TMS) |
15-30 mg/kg |
Every 12 hours (BID) |
Keratoconjunctivitis sicca (KCS), hypersensitivity reactions (polyarthritis, anemia, thrombocytopenia), hepatopathies. |
High idiosyncratic risk in certain breeds (Doberman). Schirmer test monitoring recommended during long treatments. |
Challenges and Adverse Effects
Treatment of MRSP infections with “reserve” antibiotics is not without difficulties.
- Chloramphenicol: Although often effective in vitro, it is associated with a very high incidence of gastrointestinal side effects (vomiting, diarrhea, anorexia), which frequently lead to treatment discontinuation. Cases of reversible neurotoxicity (posterior limb paresis) have also been described.
- Doxycycline/Minocycline: Clinical failures have been reported despite favorable sensitivity tests. This may be due to sensitivity interpretation thresholds (breakpoints) that do not always accurately predict clinical efficacy for S. pseudintermedius.
- Rifampin: Must be used in combination with another effective antibiotic to prevent rapid selection of resistant mutants. Liver function monitoring is recommended due to the risk of hepatotoxicity.
- Aminoglycosides (e.g., amikacin): These drugs require injection administration and careful monitoring of renal function due to their potential nephrotoxicity.
C. Management of the Underlying Cause: The Key to Preventing Recurrences
Antimicrobial treatment, whether topical or systemic, only addresses the consequences of the disease (bacterial infection) and not its cause. Without rigorous diagnosis and control of the underlying primary disease (atopic dermatitis, food allergy, hypothyroidism, etc.), recurrences of pyoderma are inevitable. Each recurrence requires a new treatment cycle, increasing selection pressure for resistance and the risk of emergence of even more difficult-to-treat strains. Long-term management of these primary conditions is therefore the most effective strategy to break this cycle, improve the animal’s quality of life, and reduce overall dependence on antibiotics.
Colonization versus Infection: Clinical and Epidemiological Implications
The distinction between colonization and infection by an MRS is a fundamental concept whose understanding is essential for rational patient management and transmission prevention. These two states represent very different biological and clinical scenarios, with distinct implications in terms of treatment and public health.
Definition and Distinction
- Colonization (or Carriage): This state is defined by the presence and multiplication of a bacterium, such as MRSP, on the body surfaces of a host (skin, nasal, oral, anal mucous membranes) without clinical signs of disease or inflammatory response from the host. The animal is a “healthy carrier“; the bacterium is part of its surface microflora, transiently or persistently, but does not cause damage.
- Infection: Infection occurs when the bacterium crosses the host’s defense barriers, invades tissues and multiplies, triggering an inflammatory response and causing clinical signs of disease (for example, pustules and inflammation of pyoderma).
Clinical and Epidemiological Significance of Carriage
Carrier status, although asymptomatic, has profound clinical and epidemiological consequences. The persistence of colonization after resolution of clinical infection is a particularly concerning phenomenon that redefines the notion of “cure.” An animal whose skin lesions have disappeared may appear clinically cured, but it may remain an asymptomatic carrier. This “hidden” carrier status transforms the cured animal into a latent epidemiological threat. It becomes a “Trojan horse,” capable of reintroducing the pathogen into the clinical environment, contaminating other vulnerable patients, and serving as a reservoir for its own future reinfection. This finding imposes a change of perspective: therapeutic success can no longer be judged solely on the basis of clinical sign resolution. It must integrate a long-term management strategy for carrier status, focused on hygiene, strict control of underlying disease to strengthen the skin barrier, and clear communication with the owner about persistent risks.
- Persistence after Treatment: One of the most important findings is that clinical cure of MRSP pyoderma does not necessarily lead to eradication of the bacterium from the organism. A follow-up study showed that among dogs initially diagnosed with MRSP pyoderma, 45.2% were still carriers of MRSP on their skin and 47.6% on their mucosal carriage sites (nose, rectum) after complete resolution of clinical signs.
- Source of Endogenous Reinfection: A colonized animal becomes its own reservoir for future infections. The bacterium resides on mucous membranes and can easily be transferred to the skin during grooming or licking. If the animal’s skin barrier is compromised again (for example, during an allergic flare), the bacterium can rapidly proliferate and cause new infection.
- Source of Exogenous Transmission: Asymptomatic carrier animals continuously disseminate MRSP in their environment (home, parks, veterinary clinics), contaminating surfaces and acting as a silent source of transmission to other animals and, potentially, to humans.
- Nosocomial Acquisition of Colonization: The clinical environment is a high-risk place for acquiring carrier status. The same study revealed that among dogs that did not have MRSP at the beginning of treatment for their pyoderma (caused by a sensitive strain), 28.3% became colonized by MRSP on their skin after treatment. The rate of mucosal carriage in this group increased significantly, from 7.8% before treatment to 26.7% after. This suggests that the hospital environment and treatment procedures can facilitate transmission and colonization by resistant strains.
Decolonization Approach
The question of whether to actively attempt to eliminate carrier status (decolonization) is complex and controversial.
- General Non-recommendation: For most cases, active decolonization of healthy carrier animals is not recommended.
- Justification:
- Colonization by MRSA (S. aureus) in companion animals is often transient and eliminates naturally within a few weeks, provided the source of re-exposure (usually human) is controlled.
- The use of systemic antibiotics to attempt to eradicate carriage is not only ineffective (antibiotics do not reach sufficient concentrations on mucosal surfaces), but it is also counterproductive as it exerts additional selection pressure in favor of resistance.
- Although topical therapies (antiseptic shampoos, nasal ointments) may temporarily reduce bacterial load, their long-term efficacy for complete carriage eradication has not been demonstrated in controlled studies.
Currently, there is no scientific consensus to recommend systematic decolonization attempts for these carrier animals. The studied protocols have shown limited and inconstant efficacy, with a non-negligible risk of selecting even more resistant strains. The preferred approach for managing colonized animals therefore focuses on non-antibiotic measures: strengthening hygiene (regular antiseptic baths to reduce skin load), strict control of underlying disease to maintain a healthy skin barrier, and implementation of infection control measures to limit transmission.
Zoonotic Potential and Transmission Prevention
The presence of multidrug-resistant bacteria in companion animals, which share our most intimate environment, raises legitimate public health questions. Assessing zoonotic risk and implementing infection control measures are shared responsibilities of veterinarians and animal owners.
A. Zoonotic Risk Assessment
It is crucial to differentiate the risk associated with different staphylococcal species, as it is not uniform.
- MRSP vs MRSA Comparison:
- Methicillin-Resistant S. aureus (MRSA): The zoonotic risk associated with MRSA is well established and considered significant. MRSA is a major human pathogen, and companion animals most often act as collateral victims of the human epidemic, becoming colonized by strains of human origin (reverse zoonosis). Once colonized or infected, however, they can become silent reservoirs within the household, contributing to MRSA persistence in the community and posing a risk of (re)infection for humans. Transmission can occur in both directions.
- Methicillin-Resistant S. pseudintermedius (MRSP): The direct zoonotic risk of MRSP is considered low. S. pseudintermedius is a bacterium highly adapted to the canine host and is poorly equipped to durably colonize or cause infections in healthy humans. However, this risk, although low, is not zero. Cases of human infections with MRSP have been reported, generally in immunocompromised individuals or those in very close contact. The main risk of MRSP for human health could be indirect: it can act as a reservoir of resistance genes (such as the mecA gene) that could potentially be transferred to more virulent human pathogens like S. aureus.
- Asymmetric Transmission Dynamics:
- For MRSA: The main reservoir is human. Companion animals are most often “collateral victims.” The predominant transmission route is Human → Animal.
- For MRSP: The main and almost exclusive reservoir is the dog. Transmission is primarily inter-canine. Transmission to humans, although possible, remains a rare event (occasional zoonotic spillover).
- Evidence of Transmission:
- Animal-Human Transmission: Several studies have used molecular typing to prove transmission of identical MRSP strains between infected dogs and their owners.
- Occupational Risk for Veterinary Personnel: Veterinary personnel constitute a population at risk for occupational carriage. A study conducted at a veterinary dermatology conference revealed a nasal carriage rate of MRSP of 3.9% among participants.
B. Infection Control Measures
Prevention of MRS transmission relies on rigorous application of hygiene and biosecurity protocols, both in clinical settings and at home.
Recommendations for Veterinary Clinics
Each veterinary clinic should implement a formal infection control program. Fundamental measures, based on “Standard Precautions,” include:
- Hand Hygiene: This is the simplest and most effective measure to prevent cross-transmission.
- Personal Protective Equipment (PPE): Wearing gloves is mandatory when handling any animal with skin lesions. Protective gowns or aprons must be worn.
- Patient Management: Hospitalized patients with confirmed or suspected MRS infection must be placed in isolation. For outpatient consultations, it is recommended to schedule these patients at the end of the day.
- Environmental Cleaning and Disinfection: High-contact surfaces and reusable equipment must be meticulously cleaned and disinfected between each patient.
Advice for Owners of Infected or Colonized Animals
Owner education is an essential component. Advice must be clear, practical, and non-alarmist.
- Rigorous Hand Hygiene: Owners must be instructed to wash their hands systematically after touching their animal.
- Limitation of Close Contacts: During the active phase of infection, it is prudent to limit intimate contacts (face licking, sleeping in bed).
- Protection of At-Risk Individuals: If immunocompromised individuals, very young children, elderly people, or individuals with skin wounds live in the household, additional precautions are necessary.
- Domestic Environment Hygiene: The animal’s bedding, toys, and food bowls must be washed regularly with hot water and soap.
Conclusion: Toward Integrated Management through Antimicrobial Stewardship and the “One Health” Approach
The emergence of methicillin-resistant staphylococci, and particularly MRSP, in veterinary dermatology, constitutes much more than a simple therapeutic challenge. It represents a complex threat that highlights the flaws in our past approaches to antimicrobial use and underscores the deep interconnection between animal health, human health, and the environment. Effective management of this problem can no longer rely solely on the search for a new “miracle” antibiotic, but requires an integrated, holistic, and sustainable approach, based on the principles of antimicrobial stewardship and the “One Health” concept.
Summary of Importance
MRS have transformed canine pyoderma, once a routine condition, into a potentially chronic, difficult-to-treat, and costly disease. The importance of MRSP does not lie in increased virulence, but in its ability to escape our first-line therapies, leading to therapeutic failures, prolonged morbidity, and increased transmission risk. The persistence of colonization after clinical cure creates silent reservoirs that perpetuate the cycle of infection and contamination. Furthermore, the zoonotic potential, although variable according to staphylococcal species, places the management of these infections at the crossroads of veterinary medicine and public health.
Principles of Antimicrobial Stewardship
The fight against the spread of antimicrobial resistance inevitably involves more responsible and judicious use of antibiotics. Antimicrobial stewardship is a coordinated approach aimed at promoting appropriate use of antimicrobials to improve patient outcomes while minimizing unintended consequences. In veterinary dermatology, this translates into concrete actions:
- Prioritize topical therapy: Elevate topical therapy to first-line treatment for all superficial pyoderma.
- Diagnose before treating: Systematically use cytology to confirm infection and reserve systemic antibiotic therapy for cases where it is absolutely necessary.
- Treat based on evidence: Base the choice of any systemic antibiotic on antibiogram results.
- Treat the cause, not just the symptom: Identify and rigorously manage the underlying disease to prevent recurrences.
- Respect protocols: Use the right dose, the right duration, and the right route of administration.
The Imperative of a “One Health” Approach
The problem of MRSP in canine dermatology is a microcosm of the global antibiotic resistance crisis. It perfectly illustrates why a compartmentalized approach is doomed to failure. Antimicrobial resistance is a problem that knows no boundaries between species or ecosystems. Antibiotic use in a dog in a veterinary clinic can have repercussions that extend to the health of people in its household. An effective “One Health” approach requires active collaboration between physicians, veterinarians, and public health officials to implement integrated resistance surveillance, harmonize guidelines, educate the public, and promote research.
Future Perspectives
The future of MRS management in veterinary dermatology will depend on our ability to fully integrate these principles into daily practice. Future development axes include strengthening epidemiological surveillance, developing and validating new non-antibiotic therapeutic strategies, and continuous improvement of education. Future research axes must focus on several complementary fronts:
- Development of alternative therapies: Research must intensify to develop and validate “post-antibiotic” approaches. These include the use of bacteriophages (bacteria-killing viruses), antimicrobial peptides, plant extracts, bacterial wall-lysing enzymes (endolysins), or molecules targeting virulence factors rather than bacterial growth.
- Antimicrobial stewardship optimization: Robust and continuous epidemiological surveillance systems are needed to monitor in real-time the evolution of MRSP clones and their resistance profiles at local and national levels.
- Understanding the commensal-pathogen transition: Better understanding of the factors that trigger the transition of S. pseudintermedius from a harmless commensal state to that of an infectious agent is a promising research avenue for preventing infection incidence.
The challenge is significant, but a proactive, collaborative, and science-based approach can allow us to preserve the effectiveness of our antimicrobials and protect the health of our animal patients and the human community as a whole.
References
- Frank, L. A., & Loeffler, A. (2012). Meticillin-resistant Staphylococcus pseudintermedius: clinical challenge and treatment options. Veterinary Dermatology, 23(4), 283-e56.
- van Duijkeren, E., Catry, B., Greko, C., Moreno, M. A., Pomba, C., Pyörälä, S., Ružauskas, M., Sanders, P., Threlfall, E. J., & Torren-Edo, J. (2011). Review on methicillin-resistant Staphylococcus pseudintermedius. Journal of Antimicrobial Chemotherapy, 66(12), 2705-2714.
- Morris, D. O., Boston, R. C., O’Shea, K., & Rankin, S. C. (2010). The prevalence of carriage of meticillin-resistant staphylococci by veterinary dermatology practice staff and their respective pets. Veterinary Dermatology, 21(4), 400-407.
- Cohn, L. A., & Middleton, J. R. (2010). A veterinary perspective on methicillin-resistant staphylococci. Journal of Veterinary Emergency and Critical Care, 20(1), 31-45.
- Duquette, R. A., & Nuttall, T. J. (2004). Methicillin-resistant Staphylococcus aureus in dogs and cats: an emerging problem? Journal of Small Animal Practice, 45(12), 591-597.
- Papich, M. G. (2023). Antimicrobial agents in small animal dermatology for treating staphylococcal infections. Journal of the American Veterinary Medical Association, 261(S1), S130-S139.
- Griffeth, G. C., Morris, D. O., Abraham, J. L., Shofer, F. S., & Rankin, S. C. (2008). Screening for skin carriage of methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Veterinary Dermatology, 19(3), 142-149.
- Lilenbaum, W., et al. (2024). Nationwide analysis of methicillin-resistant staphylococci in cats and dogs: resistance patterns and geographic distribution. American Journal of Veterinary Research.
- Pomba, C., Rantala, M., Greko, C., Baptiste, K. E., Catry, B., van Duijkeren, E., Mateus, A., Moreno, M. A., Pyörälä, S., Ružauskas, M., & Torren-Edo, J. (2017). Public health risk of antimicrobial resistance transfer from companion animals. Journal of Antimicrobial Chemotherapy, 72(4), 957-968.
- Hillier, A., Lloyd, D. H., Weese, J. S., Blondeau, J. M., Cossens, C., Guardabassi, L., Papich, M. G., & Frank, L. (2014). Recommendations for approaches to meticillin-resistant staphylococcal infections of small animals: diagnosis, therapeutic considerations and preventative measures. Veterinary Dermatology, 25(3), e39-e53..
- Ngassam, C. T., et al. (2025). Better treating pyoderma requires better recognition and knowing how to detect bacterial resistance to antibiotics. Le Nouveau Praticien Vétérinaire canine-féline.
- Gmyterco, V. C., Luciano, F. B., Ludwig, L. A., Evangelista, A. G., Ferreira, T. S., Borek, F., & de Farias, M. R. (2025). Comparative study of a commercial formula containing natural antimicrobials versus oral cephalexin or topical chlorhexidine-miconazole therapies for treating superficial pyoderma in dogs. Veterinary Dermatology.
- Perreten, V., Kadlec, K., Schwarz, S., Grönlund Andersson, U., Finn, M., Greko, C., Moodley, A., Kania, S. A., Frank, L. A., Bemis, D. A., Franco, A., Iurescia, M., Battisti, A., Duim, B., Wagenaar, J. A., van Duijkeren, E., Weese, J. S., & Guardabassi, L. (2010). Clonal spread of methicillin-resistant Staphylococcus pseudintermedius in Europe and North America: an international collaborative study. Journal of Antimicrobial Chemotherapy, 65(6), 1145-1154.
- Loeffler, A., & Lloyd, D. H. (2010). Companion animals: a reservoir for methicillin-resistant Staphylococcus aureus. Journal of Antimicrobial Chemotherapy, 65(4), 594-601.
Podcast