An 8-month-old female spayed domestic short hair was presented with a chronic history nasal congestion, coughing, nodules on her head and muzzle area, in addition to mucoid ocular and nasal discharge.
Andrew Simpson, DVM, MS, DACVD
VCA Aurora Animal Hospital
July 2024
History
An 8-month-old female spayed domestic short hair was presented with a chronic history nasal congestion, coughing, nodules on her head and muzzle area, in addition to mucoid ocular and nasal discharge. The cough was described as dry and non-productive. The cat has become increasingly lethargic with a decreased appetite. There is no history of vomiting or diarrhea, and no history of fever. Urination and water consumption were reported to be normal. She is completely housed indoors with no other animals. FeLV and FIV status are both negative. There was no response to previous trials of doxycycline, famciclovir, or L-lysine. Thoracic radiographs showed no significant findings.
General Exam
– Temperature 102.8° F
– Heart rate: 170 beats per minute
– Respiratory rate: 40 breaths per minute
– Capillary refill time < 2sec
– Mucus membrane color: Pale pink
– Body Condition Score: 4/9
– Body Weight: 2.65 kg
– Thoracic auscultation: no murmurs or arrhythmias noted, lungs auscult clearly in all fields.
– No active nasal discharge, but mild swelling of the bridge of the nose
– Mild chemosis of both eyes
– Submandibular lymph nodes enlarged bilaterally. Popliteal lymph nodes are enlarged and firm bilaterally. Remainder of peripheral lymph nodes not appreciated.
Dermatologic Exam
Multiple erythematous nodules with erosions/ulcerations present (measuring 3-7 mm) on the right eyelid margin, pre-auricular regions bilaterally, dorsal nasal planum, and dorsal head.
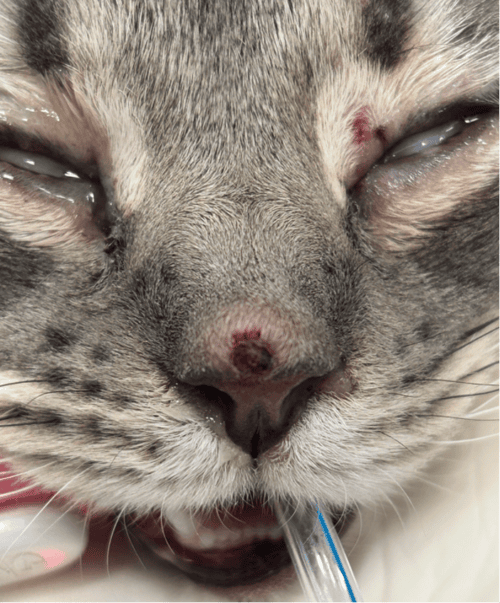
Figure 1: A 0.5 cm dermal nodule with erosion and mild hemorrhage present on the bridge of the nose. Note additional erosion on the superior eyelid of the left eye.
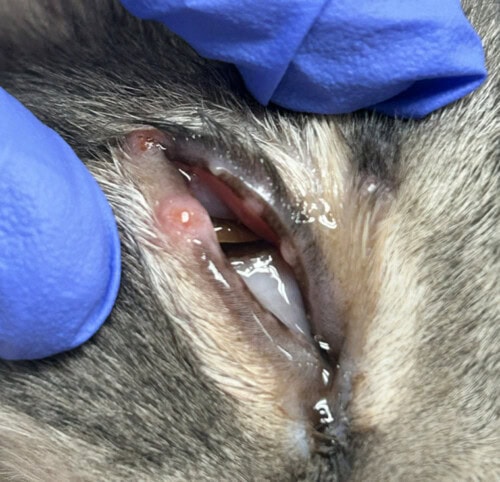
Figure 2: Multiple erythematous nodules present along the eyelid margins of the right eye.

Figure 3: Multiple, small alopecic nodules with mild erosion on the dorsal head.
Diagnostics
Bloodwork, urinalysis, and diagnostic imaging were performed to assess any systemic disease. To evaluate the organomegaly noted on abdominal ultrasound, fine needle aspirate cytology was sampled from the spleen and the liver. Biopsy of the nasal planum and nasal mucosa were submitted for histopathology, tissue culture (aerobic, anaerobic, and PCR analysis. Urine fungal testing was also submitted to evaluate potential systemic fungal disease.
The results are as follows:
Bloodwork and Urinalysis:
- Complete blood cell count: No significant findings
- Serum chemistry: No significant findings
- Urinalysis: USG 1.055, pH 6.0, Protein 2+
Diagnostic Imaging:
- Thoracic radiographs: Radiographically normal thorax without pulmonary metastasis
- Abdominal ultrasound: mild to moderate splenomegaly, hepatomegaly, and medial iliac lymph node enlargement
- Nasal CT: Bilateral rhinitis and frontal sinusitis with mild turbinate destruction caudodorsally on the right side, as well as nasal, right maxillary, and frontal bone osteolysis bilaterally. Bacterial rhinitis cannot be ruled-out, but mycotic rhinitis may also be considered. Bilateral mandibular and medial retropharyngeal lymph node enlargement.
Microbiology Findings:
- Feline Respiratory PCR Panel: negative
- Histoplasma Quantitative Sandwich Enzyme Immunoassay(antigen detection): above the limits of quantification
- Cryptococcal Latex Agglutination: negative
- Coccidioides Immunodiffusion Assay (IgG and IgM): negative for both
- Aerobic culture of the skin: no growth
- Aerobic and Anaerobic culture of the nasal mucosa: no growth of significant organisms
- Fungal culture of the skin: no growth
- Fungal culture of the nasal mucosa: no growth
Cytology Findings:
- Impression smear cytology of nasal planum: pyogranulomatous inflammation
- Cytology (liver): Histoplasmosis with mild pyogranulomatous inflammation.
- Cytology (spleen): Histoplasmosis with mixed cell inflammation.
- Cytology (popliteal and mandibular lymph nodes): Histoplasmosis with mild to moderate pyogranulomatous lymphadenitis.
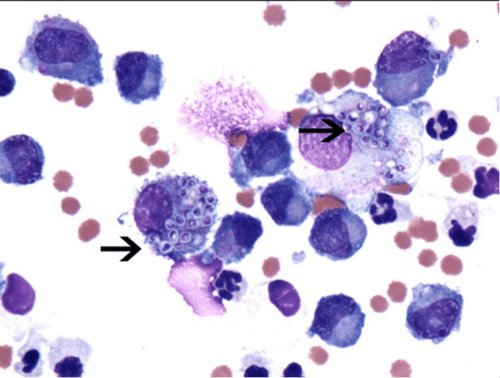
Figure 4: Splenic Aspirate. Note the presence of neutrophils, macrophages, and lymphocytes with intracellular round-to-oval shaped yeast organisms (consistent with Histoplasma capsulatum) with a purple nucleus, lightly basophilic protoplasm surrounded by a thin, clear halo.
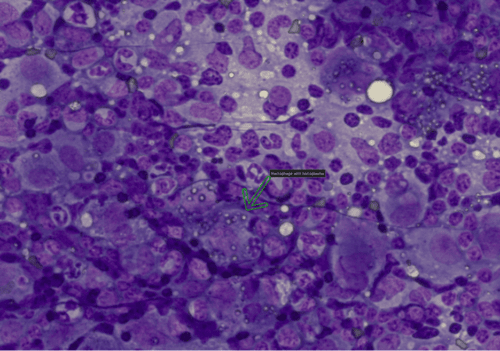
Figure 5: Popliteal Lymph Node. large numbers of intracellular yeast organisms consistent with Histoplasma capsulatum.
Histopathology Findings:
- Histopathology (skin): nodular histiocytic to granulomatous dermatitis with intralesional yeast (histoplasmosis)
- Histopathology (nasal): Moderate pyogranulomatous rhinitis.
- Special Stains (nasal): The special stains for fungal organisms confirmed the presence of spherical yeast structures consistent with Histoplasma capsulatum. Special stains for bacteria and acid-fast bacteria are negative.
Assessment
Based on the positive identification of fungal organisms morphologically compatible with Histoplasma capsulatum found in the skin, nasal mucosa, spleen, liver, and lymph nodes on cytology and histopathology, the definitive diagnosis was determined to be disseminated histoplasmosis. In addition, positive antigen levels of Histoplasma supported this diagnosis.
Treatment Plan
Oral itraconazole was prescribed (10 mg/kg PO q24 h) in addition to a tapering course of oral prednisolone (1 mg/kg q24 h for 7 days, then 0.5 mg/kg q24h for 7 days, then 0.5 mg/kg every other day for 7 days) to reduce the intense inflammatory response oftentimes seen from dying fungal organisms. To help in potentially killing fungal organisms along with facilitating the healing process, hyperbaric oxygen therapy was performed in 6 separate sessions, maintaining 14.7 PSI for 45 minutes per session.
Follow-up
After 3 months of daily itraconazole, the Histoplasma antigen by quantitative enzyme immunoassay was negative. This test was repeated 1 month later and found to be negative again. Itraconazole was continued at the same daily dose until 1 month after the second negative assay. All dermatologic lesions completely resolved in addition to the respiratory signs.

Figure 6: Complete resolution of all cutaneous lesions after 4 months of oral, daily itraconazole therapy.
Discussion
Histoplasmosis in cats is caused by a dimorphic saprophytic soil fungus, Histoplasma capsulatum. Organisms are more commonly found in decaying wood and soil containing nitrogen-rich organic matter such as bird or bat excrement. Geographically, Histoplasma is found in the central United States in the Ohio, Mississippi, and Missouri River Valleys, although worldwide distribution has been documented in Australia, Brazil, Italy, and Japan. Histoplasmosis is not reported to be zoonotic or contagious among cats.
Most cats become infected via inhalation, after which point yeast are phagocytized and disseminated systemically via blood vessels and lymphatics. Less commonly, localized cutaneous infection can occur via direct inoculation. Disseminated disease is more common vs localized cutaneous infection in cats.
Among cutaneous and systemic fungal diseases in cats, histoplasmosis is considered less common. Most cats with this disease are between 4-9 years of age, with Persian cats overrepresented. Clinical signs in cats typically consist of fever, decreased appetite, dyspnea, weight loss, skin lesions, and ocular lesions, with 40% of cats showing respiratory signs. Papules, nodules, ulcers, and draining tracts are more commonly found on the nose, pinnae, and face but can occur anywhere on the body. Bone and joint involvement have also been reported more recently in cats. Disseminated disease including lymph nodes, spleen, liver, and eyes. Gastrointestinal disease can develop in cats with histoplasmosis, though less commonly than dogs.
Fungal organisms can be identified on aspirate cytology from affected organs (i.e. skin nodules, lymph nodes, liver, spleen), to reveal pyogranulomatous inflammation as well as round yeast bodies measuring 2.4 micrometers in diameter with a basophilic center and lighter halo. Histopathology of affected tissue can also confirm the presence of infection. Culture of the organisms can be performed, however, risk of exposure to laboratory personnel should be strongly considered before submitting tissue for fungal culture.
Antigen testing (enzyme immunoassay) in urine, plasma, serum, CSF, and bronchoalveolar lavage is commercially available. This is a highly sensitive test and currently considered the most recommended molecular testing for identifying Histoplasmosis in cats. It can be used for initial diagnosis in addition to monitoring therapeutic response. Antifungal treatment should be continued until negative antigenemia has been achieved.
Antibody serology is available through semi-quantitative indirect IgG antibody enzyme immunoassay in blood serum. This should be considered if clinical suspicion is high for histoplasmosis but the antigen enzyme immunoassay is negative. Efficacy of diagnosis is increased when combining both the antigen and antibody tests.
Itraconazole is currently the treatment of choice for histoplasmosis in cats. Amphotericin B can be used in combination for refractory cases. Fluconazole and ketoconazole have been shown to be inferior drugs for feline histoplasmosis. Treatment for histoplasmosis may take 6-12 months or longer, depending on response and results from diagnostic monitoring. The prognosis for most cats is fair to good with prolonged antifungal treatment.
References
- Fielder SE, Meinkoth JH, Rizzi TE, Hanzlicek AS, Hallman RM. Feline histoplasmosis presenting with bone and joint involvement: clinical and diagnostic findings in 25 cats. Journal of Feline Medicine and Surgery. 2019;21(10):887-892.
- Lloret A, Hartmann K, Pennisi MG, et al. Rare systemic mycoses in cats: blastomycosis, histoplasmosis and coccidioidomycosis: ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery. 2013;15(7):624-627.
-
Palha de Brito Jardim M, Hanzlicek AS, Cid GC, Makita MT, Souza HJ. Histoplasmosis in domestic cats: new minimally invasive diagnostic techniques. Journal of Feline Medicine and Surgery. 2024;26(6).