Dermatozoonoses are a diverse group of skin conditions transmissible between vertebrate animals and humans. These pathologies, although relatively rare compared to all known zoonoses, represent a significant health challenge, particularly in the urban context where close proximity with pets is intensifying.
The veterinary practitioner, at the interface between animal and human health, plays a crucial role in the detection, treatment, and prevention of these conditions.
The close relationship between humans and animals, while providing undeniable psychological and social benefits, also exposes owners and professionals in contact with animals to various transmissible pathogens. These dermatozoonoses, of mycotic, parasitic, bacterial, or viral origin, require particular vigilance and a collaborative approach between physicians and veterinarians for optimal patient care, whether they are two-legged or four-legged.
Definition and classification of dermatozoonoses
Dermatozoonoses represent a specific subset of the vast field of zoonoses. They are defined as diseases or infections naturally transmissible from vertebrate animals to humans, and vice versa, clinically manifesting in humans as skin lesions, with the exception of generalized allergic reactions. This term derives from the Greek roots “zoo” (animal) and “nosos” (disease), conceptualized by Virchow in the 19th century, then refined by the World Health Organization in 1959.
In urban environments, the main vertebrates responsible for these conditions are pets – domestic carnivores such as dogs and cats, but also rodents and lagomorphs increasingly present in our homes. Exposed populations include not only pet owners, but also various at-risk professions: veterinarians and their staff, breeders, groomers, and other professionals in regular contact with these animals.
Classification according to transmission methods
The World Health Organization proposes a classification of zoonoses into four distinct categories, based on their transmission methods:
- Orthozoonosis or direct zoonosis: The causative agent requires only one vertebrate species for its maintenance, although it can affect several species. This species allows transmission to humans. The majority of classic infectious zoonoses such as rabies, anthrax, or brucellosis belong to this category.
- Cyclozoonosis: In this case, the biological cycle involves several vertebrate species, but only one of them is responsible for human contamination. Echinococcosis perfectly illustrates this process, with its cycle involving dogs and herbivores, where the dog acts as a contaminant to humans.
- Metazoonosis: This category requires passage through an invertebrate, usually an arthropod, which allows transmission to humans. Arboviruses like yellow fever (maintained on monkeys and marsupials then transmitted to humans by a mosquito), rickettsioses and leishmaniasis fall into this classification.
- Saprozoonosis: These conditions require the passage of the causative agent into the external environment. Fascioliasis illustrates this mechanism, with the maturation of cercariae responsible for swimmer’s itch.
This systematic classification allows for a better understanding of epidemiological cycles and, consequently, promotes the implementation of prevention strategies adapted to each type of transmission.
Dermatozoonoses of mycotic origin
Fungal infections transmissible between animals and humans constitute an important chapter of dermatozoonoses. These conditions, mainly represented by dermatophytoses and sporotrichosis, deserve particular attention due to their prevalence and potential impact on human health.
Dermatophytoses
Dermatophytoses, commonly called “ringworm”, are superficial infectious mycoses caused by epidermotropic, keratinophilic, and keratinolytic fungi – dermatophytes. These fungi have the particularity of feeding on keratin, a protein constituent of superficial structures like hair, fur, nails, and the stratum corneum of the epidermis.
Zoophilic dermatophytes, of animal origin, are a major source of dermatozoonoses. Three main species are to be considered:
- Microsporum canis: The main reservoir is the cat (more rarely the dog, especially the Yorkshire terrier breed, and other small mammals). This species is responsible for approximately 95% of feline dermatophytoses and 65% of canine cases.
- Trichophyton mentagrophytes: Its reservoir consists of rodents, lagomorphs, and equids. The percentage of asymptomatic carriers varies considerably depending on the species: approximately 15% in guinea pigs, 10 to 40% in rabbits, and nearly 50% in mice and rats.
- Trichophyton verrucosum: Mainly found in ruminants.
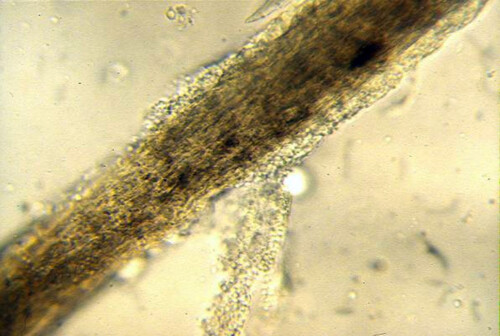
Ringworm hair
Most dermatophytes show a close adaptation to a target species, in which they generally cause few clinical signs. In cats, for example, the incidence of asymptomatic carriers of M. canis is around 10%, but can reach much higher percentages in stray cats or certain breeds like Persians. Alarmingly, approximately 50% of people in contact with an infected cat, whether asymptomatic carrier or clinically ill, develop skin lesions.
Clinical presentation in animals
In cats, the classic lesions of M. canis are characterized by single or multicentric, erythematous, non-pruritic, scaly alopecia, with centrifugal extension. Preferred locations are the face, ears, and paw extremities. A late diagnosis may reveal loco-regional or generalized alopecia, sometimes accompanied by pruritic inflammation. In some cases, only a dorso-lumbar keratoseborrheic condition is observable. Onychomycoses with M. canis are rare, while mycetomas are found exclusively in immunocompromised individuals and in the Persian breed.
In dogs, in addition to M. canis, three other species are found: T. mentagrophytes, M. persicolor (both zoophilic agents) and M. gypseum (geophilic agent). Certain breed predispositions exist: Yorkshire terriers seem particularly vulnerable to generalized ringworm with M. canis, while terriers (Fox terrier and other terrier hunting dogs) show increased susceptibility to facial dermatophytoses caused by T. mentagrophytes and M. persicolor.
The majority of affected dogs present classic lesions: single or multicentric, rounded, slightly scaly alopecia, with centrifugal evolution, generally not very pruritic. Preferred locations are the face and the distal extremity of the limbs. Kerions (suppurative nodular inflammatory lesions) are regularly observed, particularly with T. mentagrophytes.
In rodents and lagomorphs, increasingly popular as pets, the almost exclusive agent is T. mentagrophytes, more rarely M. canis. Clinically, dermatophytoses manifest as centrifugal alopecia, often pruritic and very inflammatory.
Diagnosis of dermatophytoses
Diagnosis is based on a multifaceted approach:
- Wood’s lamp examination: A typical green fluorescence of contaminated hairs is observable in 50% of M. canis cases. This technique nevertheless requires some experience to distinguish specific fluorescence from artifactual fluorescence.
- Direct microscopic examination of hairs and scales: It can reveal an alteration of the hair shaft, sheathed by spore sleeves (endoectothrix type hair invasion in the case of M. canis).
- Mycological culture: It allows definitive identification of the responsible dermatophyte. In asymptomatic carriers, sampling can be performed by brushing the entire body surface with a sterile toothbrush or a sterile carpet square, then culturing on Sabouraud agar.
Treatment and prevention
Treatment is imperative, even if spontaneous healing is possible (although it can take several months or even years). Therapeutic efficacy depends on taking into account two essential factors:
- The high contagiousness of dermatophytoses
- The prolonged resistance of spores in the environment (several years)
The therapeutic approach combines:
- Topical treatment: clipping (discussed on a case-by-case basis), shampoos and baths based on enilconazole or miconazole associated with chlorhexidine
- Systemic treatment: griseofulvin, ketoconazole, itraconazole
- Environmental treatment: enilconazole or bleach
Treatment should be continued until mycological cure confirmed by negative cultures, with two negative controls spaced one month apart. In breeding, the major problem remains permanent recontamination due to the high spore load in the environment, making eradication practically impossible despite sanitary breaks. Ideally, in disease-free breeding facilities, any new animal should be quarantined until a negative mycological culture is obtained.
Impact on humans
In humans, dermatophytoses of animal origin are characterized by remarkable clinical polymorphism:
- Erythematosquamous lesions of the scalp (mainly due to M. canis)
- Circular erythematosquamous lesions of glabrous areas, often polycyclic with a vesiculosquamous border (M. canis, T. mentagrophytes)
- Crusted and painful lesions with severe inflammatory reaction (kerion) of the scalp, nape of the neck, or beard (sycosis), mainly caused by T. mentagrophytes, more rarely M. canis
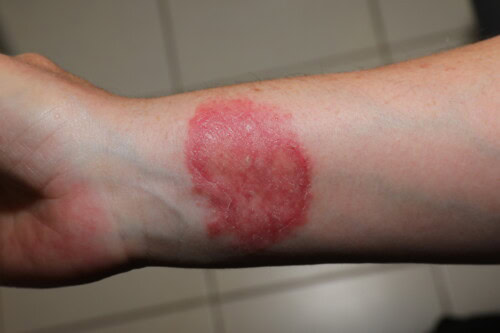
Human contamination during ringworm
It is important to note that ringworm is listed in schedule No. 46 of occupational diseases (cutaneous mycoses). According to the Labor Code and European directives, the employer’s obligations relate to the prevention of occupational risks, specific regulatory measures, hygiene and safety measures, as well as medical surveillance.
Sporotrichosis
Sporotrichosis is a deep mycosis with high zoonotic potential, caused by a dimorphic fungus, Sporothrix schenckii. This pathogen has a remarkable characteristic: it exists in filamentous form in the surrounding environment (decomposing plants, humus, soil) and transforms into yeast after penetrating the host’s tissues following skin breakage.
Modes of contamination
While punctures and injuries by contaminated plant elements are the classic mode of contamination, the infected cat represents a particularly worrying source of contamination. Indeed, pet owners, veterinarians, and their staff are exposed to significant risk through contact between their pre-existing skin lesions and exudates when handling the sick animal.
A distinctive characteristic of this infection is the difference in fungal load depending on the host species: in dogs or humans, the fungal agent is present in limited quantities in the lesions, while in cats, fungal elements reach extremely high concentrations. Transmission to humans mainly occurs through skin trauma: bite or scratch from a contaminated cat.
It should be noted that canine sporotrichosis, rarer than its feline counterpart, has so far not been associated with proven cases of human contamination.
Clinical presentation in animals
In dogs and cats, three distinct clinical forms have been described, with a variable incubation period of one week to two months:
- Cutaneo-lymphatic form (80% of cases): It is characterized at the inoculation site by the progressive development of a single nodule, initially asymptomatic then ulcerated, located on the face or a paw extremity. Other ulcerated and fistulous nodules may appear along the lymphatic vessels.
- Strictly cutaneous form (less frequent): Preferred locations are the paw extremities, which present nodular lesions or areas of alopecia with raised, ulcerated and crusted edges.
- Generalized form: Observed mainly in immunocompromised cats, it results from the hematogenous dissemination of infectious spores.
Diagnosis and treatment
Diagnosis is based on cytological examination of skin smears and biopsies, which reveal a remarkable abundance of fungal elements in cats. Mycological culture confirms the diagnosis.
Treatment is based on the administration of systemic antifungals, with a preference for itraconazole and fluconazole. In systemic forms, amphotericin B gives excellent results. Treatment should be continued for at least one month after apparent clinical resolution. In cats, in addition to systemic fungicide treatment, strict hygiene measures are essential:
- Careful cleaning of hands and arms with antifungal substances (chlorhexidine, povidone iodine)
- Mandatory wearing of protective gloves
- Clear information to owners regarding the major zoonotic risk
Due to the high risk of transmission and the prolonged duration of treatment, euthanasia may legitimately be considered for carrier cats.
Manifestations in humans
In humans, incubation varies from three weeks to three months. The cutaneo-lymphatic form, the most frequent, manifests at the inoculation site as a progressive nodule, initially asymptomatic then ulcerated, typically located on the back of the hand, a finger, a foot, or the face.
Parasitic dermatozoonoses
Cutaneous conditions of parasitic origin transmissible between animals and humans constitute a heterogeneous but important group of dermatozoonoses. These parasitic infestations, involving various mites, insects, or helminths, present varied clinical pictures in both animals and humans.
Scabies
Sarcoptic mange
Sarcoptic mange is a highly contagious acariasis due to the proliferation in the epidermis of mites of the Sarcoptidae family, Sarcoptes scabiei var. canis. Its importance in veterinary and human dermatology stems from its increasing frequency, clinical severity, and proven zoonotic potential.
The species mainly affected by Sarcoptes scabiei var. canis are dogs, foxes, and ferrets, but occasionally also cats, humans, and horses. The life cycle is characterized by its rapidity (10 to 13 days) in favorable environmental conditions. Females, extremely prolific, lay 2 to 3 eggs daily (approximately 50 eggs per female) for 2 to 4 weeks. After fertilization, they dig epidermal burrows (unlike the furrows observed with human sarcopts) to deposit their eggs.
These eggs hatch in 2-3 days, releasing hexapod larvae which develop into nymphs then adults. Sarcopts feed on epidermal tissues (histophages). Their survival in the external environment is limited to approximately 10 days, requiring specific conditions (15-25°C, relative humidity between 25 and 85%).
Sarcoptic mange remains under-diagnosed in dogs. It mainly affects young dogs under one year old, particularly in collective contexts (kennels, shelters), but can also affect adult or elderly dogs weakened by an intercurrent condition.
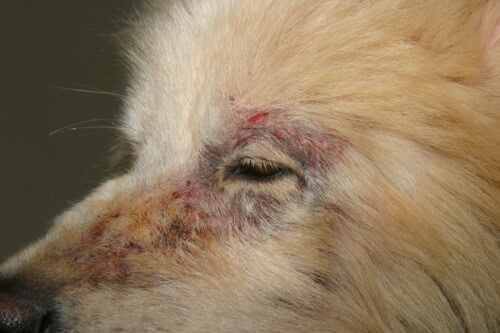
Sarcoptic mange with facial involvement
The pathogenic power of Sarcoptes scabiei var. canis is exerted through various mechanisms:
- Mechanical and chemical actions (inoculation of vasodilatory and anticoagulant proteins)
- Antigenic action (excrements, molting products, saliva)
- Induction of type I, IV, and III hypersensitivity phenomena
Type III hypersensitivity can cause immune complex deposits in various organs, including the kidneys, causing glomerulonephritis. This systemic dimension justifies considering canine sarcoptic mange as a general disease and not just dermatological.
Clinically, after a variable incubation (approximately 3 weeks post-contact), the classic picture associates:
- Intense pruritus with positive otopedal reflex
- Erythematous and papular lesions (“mange bumps”)
- Moth-eaten alopecia
- Crust formation
The lesion distribution is characteristic at the beginning of evolution, primarily affecting the face (free edges of the ear flaps), limbs (elbows), and sternum. Systemic manifestations may occur in long-standing infestation or in elderly dogs: hyperthermia, anorexia, weight loss, polyuro-polydipsia secondary to immunological glomerulonephritis.
Atypical forms are increasingly reported:
- Frustrated localized forms, not very pruritic and not very contagious
- “Norwegian scabies” characterized by thick compact scales, moderate pruritus and the massive presence of sarcopts at different evolutionary stages in skin scrapings (typically in immunocompromised animals)
The diagnosis of certainty is sometimes difficult. Microscopic examination of deep skin scrapings, performed in the predilection areas, reveals parasites or their traces (sarcopts, eggs, excrement) in only about 50% of cases. Serological diagnosis is an alternative, with a sensitivity and specificity of approximately 80-90%. Faced with strong clinical suspicion without parasitological confirmation, a trial treatment is recommended according to the principle “if you suspect it, treat it”.
Treatment includes two approaches:
- Topical acaricides (diluted amitraz)
- Systemic spot-on acaricides (selamectin or moxidectin)
The zoonotic dimension is significant, with human contaminations observed in 25 to 50% of cases of canine sarcoptic mange. Incubation in humans is 8 to 15 days, resulting in prurigo of the trunk, arms, and legs. Characteristically, no scabious burrow is observed, unlike human scabies.
This is explained by the fact that Sarcoptes scabiei var. canis cannot survive more than 15 to 20 days in humans, due to narrow host specificity. The parasite is unable to reproduce in human skin (absence of ovigerous females in the epidermis, no burrows) and remains confined to the surface without terebrant or antigenic action.
Therefore, appropriate treatment of the mangy animal usually suffices to resolve symptoms in humans – sarcoptic mange being considered a “hemizoonosis” where the parasite dies quickly in human skin without reproducing there. Persistence of human symptoms should raise suspicion of a persistent source of contamination: untreated or poorly treated animal, asymptomatic carrier conspecific not identified, or survival of parasites in the environment.
Notoedric mange
Notoedric mange is a contagious acariasis mainly affecting cats, rats, and hamsters. It is caused by the multiplication on the surface and in the epidermis of psoroptic mites of the Sarcoptidae family: Notoedres cati in cats and Notoedres muris in rodents.
Relatively rare in cats in mainland France, this condition is more common in overseas departments and territories (Reunion Island, West Indies), as well as in Italy, Slovenia, and Spain. In hamsters and rats, it is one of the most common pruritic dermatoses.
The biological cycle of notoedres is comparable to that of sarcopts, with marked contagiousness, particularly by direct contact, potentially affecting cats, dogs, and humans. This infestation can be enzootic or epizootic with significant morbidity. Sensitivity factors include young age (kittens), immunosuppression (FeLV or FIV positive cats), and, in rodents, rat or pregnant female status.
Clinically, in cats, lesions generally begin on the face (muzzle, lips, eyelids, ear flaps) before generalizing to the limbs and perianal and abdominal regions. They manifest as diffuse erythematous and scaly alopecia, rapidly progressing to crusty formations. Pruritus is usually intense.
In hamsters, cutaneous manifestations include crusty lesions preferentially located on the snout, ear flaps, and extremities of the limbs, with frequent genital involvement. Pruritus varies from moderate to intense.
In rats, lesion distribution is more restricted, limited to the free edge of the ear flaps and the snout, in the form of pseudo-tumoral verrucosities. Papulo-crusty lesions are generally observed on the tail, but generalization is unusual. Pruritus intensity varies from moderate to severe.
Human contaminations, whose precise incidence is difficult to assess, produce skin signs similar to those of canine sarcoptic mange: prurigo of the trunk, arms, and legs. As for Sarcoptes scabiei var. canis, notoedres cannot reproduce in human skin (absence of epidermal ovigerous females and burrows) and remain confined to the surface, without terebrant action or significant antigenic response. Resolution of scabies in the animal therefore generally leads to recovery in humans, this notoedric mange being also considered a hemizoonosis.
Diagnosis, relatively simple, is based on the identification of notoedres at various evolutionary stages (adults, nymphs, larvae, eggs) and their excrement during skin scrapings.
Treatment involves systemic acaricides (avermectins and milbemycins), with the need to treat all animals in the group, whether or not they present clinical signs.
Trixacaric mange
Mange due to Trixacarus caviae is a contagious acariasis specific to guinea pigs (and occasionally mice), caused by a psoroptic mite of the Sarcoptidae family. Triggers remain poorly understood, with often discreet contamination conditions and variable incubation period.
Reconstructing the history of infestation is often complex, contamination frequently preceding the acquisition of animals. These guinea pigs probably harbor a small number of parasites without clinical manifestation, until environmental changes (diet, habitat, overpopulation) or an alteration in health status cause parasitic multiplication beyond the pathogenic threshold.
Notably, the presence of severely affected animals does not systematically lead to contamination of conspecifics in contact, suggesting an individual component in susceptibility to infestation.
The clinical picture associates constant, early, and often intense pruritus with rapidly generalized lesions: erythema, papules, scales, progressing to extensive crusty formations. Distribution may be loco-regional or generalized. General condition may deteriorate during chronic evolution, with apathy, anorexia, weight loss, and sometimes fatal outcome.
Human contaminations, regularly reported, result from frequent and prolonged contact with the sick animal. Infestation episodes have notably been described in school communities, involving kindergarten children in contact with a severely affected guinea pig.
In humans, this scabies manifests as a papular pruritic dermatosis (prurigo type) mainly affecting the arms, neck, and legs. As with other animal scabies, appropriate treatment of the animal usually suffices to resolve human symptoms, this trixacaric mange also being a hemizoonosis where the parasite cannot reproduce in human skin.
The therapeutic protocol is based on the use of topical or systemic acaricides (avermectins or milbemycins), with mandatory treatment of all conspecifics. Cleaning and deacarization of the environment complete the management.
Cheyletielloses
Cheyletielloses are a set of parasitic dermatoses caused by mites of the genus Cheyletiella, belonging to the Cheyletidae family. Three main species are identified, each with a host preference: Cheyletiella yasguri (dog), Cheyletiella blakei (cat), and Cheyletiella parasitivorax (rabbit).
These mites have particular biological characteristics: adults lay eggs at the base of hairs and feed on skin debris and tissue fluids. Unlike other surface ectoparasites, cheyletiellae can sink into epidermal debris, even into the stratum corneum, forming pockets. Their mobility on the skin is remarkable.
They are obligate parasites completing their entire life cycle on their host, with a development time of approximately 35 days. Transmission mainly occurs by direct contact with clinically affected or asymptomatic carrier animals, but also indirectly via the environment, where females can survive up to 10 days. Interspecific contaminations are possible, and survival could be prolonged in favorable environmental conditions (relatively low temperature, high humidity, moderate light). Parasites can contaminate animal bedding, wall and floorboard interstices, sometimes even in the absence of animals.
Cheyletielloses mainly affect young animals (puppies and kittens from kennels or catteries), but also adult dogs (often asymptomatic carriers) and adult cats. Certain breed predispositions have been observed: dwarf canine breeds (Yorkshire Terrier, Bichon, Poodle) and, in cats, the Persian breed.
Clinical presentation varies depending on species and age:
- In puppies: intense pruritus with positive otopedal reflex and pronounced squamosis affecting the head, back, and loins
- In kittens: discreet signs limited to dorso-lumbar pityriasiform squamosis
- In adult cats: more inflammatory skin lesions with pruritic papulo-crusty dermatitis
- In rabbits: often asymptomatic infestation or pruritic and scaly dermatosis mainly trunkal
Human contamination is frequent (>50% of cases), generally occurring from C. blakei and C. yasguri. This transmission, often underestimated, mainly results from direct contact with the parasitized animal (clinically affected or asymptomatic carrier), but can also occur indirectly.
In humans, clinical manifestations appear rapidly, from the second day after contact, in the form of very pruritic papules located on the forearms, elbow creases, and arms, chest, abdomen, and thighs.
Diagnosis, generally simple, is based on the demonstration of cheyletiellae by skin scrapings, brushing or “scotch test” and microscopic observation of scales and debris. Visualization of parasites is usually easy in dogs (adults, nymphs, eggs) but more difficult in cats, where adults are rarely identified and only eggs at the base of hairs can be observed. In rabbits, parasitic forms are easily detectable.
Specific treatment combines:
- Topical or systemic acaricides (avermectins or milbemycins)
- Prolonged treatment (minimum eight weeks) due to the resistance of eggs to acaricides
- Mandatory treatment of all animals in contact
- Environmental treatment
Isolation and appropriate treatment of animals generally allow spontaneous resolution of lesions in humans, cheyletiellae not being able to reproduce in human skin.
Pulicoses
Infestations by fleas (pulicoses) are one of the most common ectoparasitoses in dogs and cats. The predominant species is Ctenocephalides felis felis – “the cat flea” – more rarely Ctenocephalides canis. Although pulicoses are not strictly considered zoonoses in the strict sense, their frequency and harmful consequences in humans justify their inclusion in this analysis of dermatozoonoses.
Fleas are apterous insects (wingless), flattened laterally. Adults, cosmopolitan and sedentary parasites, spend most of their time on the animal host. Their biological cycle has several particularities: females begin to lay their eggs on the host within 24 to 48 hours of their first blood meal. These eggs, with a smooth surface, fall into the environment where development takes place (eggs, three larval stages, nymphal stage).
The duration of the complete cycle is approximately three weeks in optimal conditions, but can be slowed down in unfavorable environmental conditions. A crucial element of epidemiology is the ability of pre-emerged adults (still in nymphal cocoons) to persist for several months in the environment, constituting a considerable parasitic reservoir. These adults emerge under the influence of specific stimuli (vibrations, light, chemical signals) and form what is commonly called “parquet fleas”.
Flea infestation is generally inapparent in healthy animals. Healthy dogs undergo moderate parasitic pressure, and the flea population is naturally limited by self-grooming behaviors (nibbling, licking) and scratching movements that expel parasites from the coat. This particularity explains the occasional difficulty in demonstrating parasites on infested animals.
The pathological consequences of pulicoses have two dimensions:
- Mechanical skin irritation due to repeated bites and movements of parasites
- The development, in some sensitized subjects, of flea bite hypersensitivity dermatitis (FBHD), which is the most common allergic dermatosis in both cats and dogs
In dogs, FBHD manifests as dorso-lumbar alopecia associating erythema, papules and crusts, with generally intense pruritus. In the absence of adequate treatment, secondary infectious complications are common. In cats, the picture is characterized by pruritic papulo-crusty dermatitis, eosinophilic plaques and/or dorso-lumbar alopecia.
Transmission to humans mainly occurs during massive infestations, when overcrowding forces fleas to change hosts. It should be specified that direct transmission of adult fleas between animals or to humans is relatively limited (10 to 15% on average). Human contamination essentially comes from young adult fleas freshly emerged from cocoons, which actively seek an available host, whoever it may be.
In humans, FBHD preferentially affects the limbs (ankles, wrists) and, in children, can extend to the trunk. The lesions, intensely pruritic, take the appearance of urticarial papules, hives, or transient vesicles. Impetigo secondary to scratching and bacterial superinfections is sometimes observed.
Diagnosis of pulicosis is based on the direct identification of adult fleas or their excrement, either with the naked eye, or by brushing with a specific flea comb. For FBHD, diagnosis is based on evocative anamnesic elements (seasonal dermatosis, presence of several dogs/cats in the environment) and compatible clinical lesions (dorso-lumbar pruritic dermatosis). Paradoxically, in animals with FBHD, visible flea infestation is often minimal.
In dogs, intradermal tests using total flea extracts can confirm FBHD by positive immediate reactions at 20 minutes and/or delayed reactions at 48 hours, although their absence does not exclude the diagnosis. These tests are considered unreliable in cats.
Treatment of pulicoses and FBHD should never be trivialized and requires a global approach:
- Elimination of fleas on the affected animal
- Mandatory treatment of conspecific animals
- Sanitation of the environment
This integrated strategy often requires close collaboration with the owner to establish an effective control program. Flea control on the animal is based on the use of residual adulticides, while management of non-parasitic stages in the environment relies on insect growth inhibitors and environmental adulticides. Complementary mechanical measures (methodical cleaning, elimination of ecological niches) are also essential.
Helminthic dermatozoonoses
Cutaneous larva migrans
The phenomenon of cutaneous larva migrans (CLM) is mainly observed in mainland France in people returning from tropical regions, where stray dogs contaminate beaches with their excrement. These dogs, generally unmedicated and not dewormed, are frequently infested with hookworms (Ancylostoma spp. and Uncinaria spp.), digestive nematodes responsible for hemorrhagic gastroenteritis in them. However, the recent description of an enzootic of larva migrans with Ankylostoma in Brittany calls for increased vigilance in mainland territory.
Cutaneous manifestations are linked to the transcutaneous penetration of L3 infective larvae. Clinically, they result in papules often crusted and pruritic, sometimes pustular, preferentially located on thin-skinned areas (abdomen) and limbs (interdigital spaces and palmar surfaces). These signs, relatively non-specific, require a precise history evoking potential contact with contaminated environments (dog living in a kennel, rural environment, hunting dog).
Beyond cutaneous manifestations, infestation can cause respiratory signs related to larval migration (bronchopneumonia), often under-diagnosed. Digestive disorders (hemorrhagic enteritis) and general signs (weight loss, anemia) are regularly observed in chronic infestations.
Sources of contamination include carrier dogs (and cats) as well as wet soils contaminated with L3 infective larvae. Poorly maintained kennels with earthen floors constitute an ideal environment for larval development, explaining the particular vulnerability of hunting dogs. Also note the possibility of indirect infestation by ingestion by dogs of small rodents that have themselves ingested L3 larvae.
Diagnosis of transcutaneous migrations is often delicate. Direct demonstration of L3 larvae by skin scrapings is rarely conclusive. Histopathological examination of skin biopsies may suggest these migrations (eosinophilic infiltrate and occasionally presence of larvae). Coproscopic examination generally easily identifies eggs, often numerous.
Prevention and control involve a multiple approach:
- Destruction of contaminated environments
- Regular and reasoned deworming, particularly of pregnant bitches (larvicidal anthelmintics)
- Reconstruction of infested dirt kennels
- Daily collection of excrement
- Intensive weekly cleaning based on cresol
In humans, the larva penetrating the skin produces a characteristic serpiginous eruption. However, this infestation constitutes a parasitic dead end, lesions generally regressing spontaneously in a few weeks to a few months.
Swimmer’s itch or furcocercarial dermatitis
Swimmer’s itch, also known as furcocercarial dermatitis (or bather’s dermatitis), is an increasingly common seasonal dermatozoonosis (June to September). This metazoonosis is caused by the epidermal penetration of larvae of a trematode, Trichobilharzia ocellata, a duck parasite, sometimes improperly called “duck flea”. Contamination occurs during freshwater bathing, particularly in lacustrine areas (Lake Annecy, Lake Bourget, Lake Geneva, Swiss and Italian lakes).
The parasitic cycle involves the trematode T. ocellata, a digestive parasite of ducks, excreted in droppings. These trematodes are then ingested by aquatic snails of the genus Lymnaea, particularly Lymnaea stagnalis. Cercariae (larval forms) are subsequently released into the water where they can contaminate ducks (definitive host) as well as humans or dogs (accidental hosts) during bathing.
Although dogs may present lesions similar to those observed in humans, it is important to emphasize that in no case can humans be contaminated from an affected dog.
In humans, the clinical picture is characterized by an intensely pruritic eruptive dermatitis, maculo-papular, located on exposed areas. The sudden appearance of papules generally occurs within 10 to 30 minutes following immersion in fresh water. Evolution is favorable, with spontaneous healing in 2 to 3 weeks, although symptomatic treatment (antihistamines or dermocorticoids) is frequently necessary.
Sanitary prophylaxis is based on interrupting the biological cycle of the parasite, involving the elimination of snails and ducks from the water bodies concerned. Since eliminating molluscs is particularly difficult, it is recommended that bathers avoid shallow water areas rich in aquatic vegetation, a privileged habitat for snails.
Leishmaniasis
Leishmaniasis is an infectious protozoonosis transmissible by inoculation, characterized by the intracellular multiplication of a flagellate protozoan, Leishmania infantum, in cells of the mononuclear phagocyte system. Its transmission is carried out by the bite of phlebotomine sandflies, with the dog being the main reservoir. In France, canine enzootic foci are mainly concentrated in the southeast, around the Mediterranean coast, from the Italian border to the Spanish border, and from sea level up to approximately 800 meters altitude.
Although the parasite has also been isolated in foxes and cats, their epidemiological role seems marginal. Recent discoveries have also highlighted other modes of transmission: syringe exchange between heroin addicts, contamination by blood products, suggesting the occasional existence of an anthropozoonotic cycle.
In dogs, leishmaniasis presents as a general disease with remarkable clinical polymorphism. Epidemiological surveys conducted in the Alpes-Maritimes and Marseille region indicate that approximately one in two leishmaniasis dogs is an asymptomatic carrier. Although systemic in this species, the condition mainly manifests as skin lesions, never isolated but associated with various clinical signs:
- Polyadenomegaly (frequent)
- Splenomegaly (rarer)
- Alteration of general condition (asthenia, facial muscle amyotrophy)
- Various complications: bilateral uveitis, arthritis, glomerulonephritis (sometimes the only clinical manifestation, with poor prognosis)
Skin lesions, typically chronic in evolution, present great morphological diversity:
- Generalized exfoliative dermatosis affecting the head, ear flaps, and limbs
- Ulcerations of paw extremities and pressure points
- Depigmentation of the nose (primary or secondary to ulcers)
- Thickening of the nose and/or paw pads
- Non-ulcerated nodules, single or multiple (particularly in certain breeds like Boxer or Dobermann)
- Generalized sterile pustular dermatitis
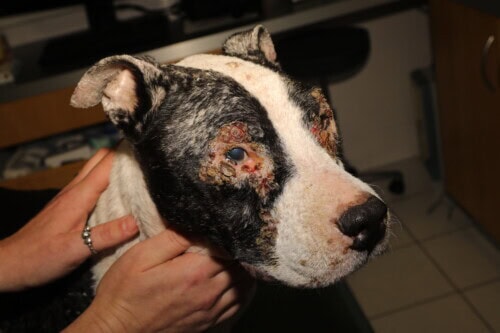
Canine Leishmaniasis
In humans, leishmaniasis presents as a systemic disease mainly affecting children and immunocompromised adults. It can also manifest in a strictly cutaneous form, with lesions located at the inoculation site, generally on uncovered areas. These nodular, ulcerated, and crusted lesions are characteristically painless, of variable size and chronic evolution.
Diagnosis in veterinary medicine is based on various techniques:
- Direct demonstration of the parasite by cytology (adenogram, myelogram)
- Serology (ELISA, indirect immunofluorescence)
- Gene amplification (PCR)
In enzootic areas, systematic annual screening is recommended, ideally performed after the exposure season (November to January), taking into account the variable but generally few months incubation period.
Management of infected dogs raises many controversies. Indeed, even treated, these animals remain carriers of the parasite. Human visceral leishmaniasis being potentially fatal in the absence of treatment, with an increasing incidence in enzootic areas, elimination of the parasitic reservoir seems logical. However, this approach would penalize responsible owners of properly medicated animals, while leaving an uncontrolled canine population. Paradoxically, systematic euthanasia of carriers, when applied, has not given the expected results and has even been accompanied by an increase in human cases.
The veterinarian plays a decisive role in informing owners about the risks and the need for rigorous monitoring, both clinical and biological.
Treatment of canine leishmaniasis is based on the combination of stibated derivatives and allopurinol, with close therapeutic monitoring. Despite owner awareness, a fraction of dogs inevitably escapes control or receives intermittent self-medication, raising the serious problem of the potential emergence of resistant strains. Consequently, veterinarians should refrain from using certain highly effective molecules such as amphotericin B, which should be reserved exclusively for human medicine for this indication.
The development of a canine vaccine would represent the ideal epidemiological control solution, but its development currently faces many obstacles.
Bacterial dermatozoonoses
Cat scratch disease or benign inoculation lympho-reticulosis
Benign inoculation lympho-reticulosis, more commonly called cat scratch disease (CSD) because of its predominant mode of transmission, is a subacute regional bacterial lymphadenopathy in humans. The causative agent, Bartonella henselae (Bartonellaceae family), was only identified in 1992. Some cases may also be attributed to Bartonella clarridgeiae. The two known genotypes (I and II) of B. henselae are involved in this condition.
B. henselae is also associated, with B. quintana, with the etiology of bacillary angiomatosis and peliosis, vasculo-proliferative diseases observed mainly in patients infected with HIV.
The cat represents the main, even the only reservoir of the bacterium. The role of the dog in carrying the infection seems very limited. Although rare, cases have been reported in the absence of direct exposure to an animal, suggesting other possible modes of transmission (flea or tick bites). Human contamination occurs in 70% of cases after scratching and in 10% after feline bites. Exceptionally, simple contact (caress, hug) could allow contamination of a pre-existing skin or mucous wound, as illustrated by the oculo-glandular form sometimes observed in people who have probably rubbed their eye after petting a cat.
Experimental infection in cats rapidly (less than a week) causes prolonged asymptomatic bacteremia, persisting for 2 to 3 months or more in some subjects (persistent recurrent bacteremia has been observed in a cat for 22 months). Some cats have remarkably high levels of bacteremia (greater than 10^6 CFU/ml of blood). Bacteremia is statistically more frequent in young cats (less than one year old). B. henselae and B. clarridgeiae may co-infect the same animal. Recently, two new species of Bartonella, B. koehlerae and B. weissii, have been isolated from cats in the United States, but their pathogenic role in CSD remains to be demonstrated.
Epidemiological studies indicate that a substantial proportion of tested cats are bacteremic, with a higher percentage among stray cats compared to domestic cats. Various surveys have revealed bacteremic cat rates ranging from 16.5% to 53% within stray feline populations, of which about a third are infected with B. clarridgeiae.
The cat flea (Ctenocephalides felis felis) plays a predominant role in the transmission of infection within this species. B. henselae can also be isolated from fleas taken from bacteremic cats. The flea excretes the bacterium in its droppings, thus contaminating the animal’s coat. Bacteria can multiply in the insect’s digestive tract and survive in its droppings. The cat contaminates its claws during grooming, thus establishing the chain of transmission to humans. Stray cats, more frequently infected, represent a source of contamination for domestic cats.
CSD is a ubiquitous disease (approximately 22,000 annual human cases estimated in the United States and 2,000 in the Netherlands in 2003) that can affect all ages, but mainly affects children and young adults. Half of the cases are reported in children under 15 years old. Bacillary angiomatosis, a severe form of the disease, is mainly diagnosed in immunocompromised adults (particularly HIV+ patients). CSD generally occurs sporadically, but small family epidemics are sometimes described.
The classic clinical picture begins with progressive adenopathy. At the inoculation site, a papule appears within a week progressing to a vesico-pustule. In more than 90% of cases, this initial lesion, which heals in 1 to 3 days, goes unnoticed. It is generally 2 to 3 weeks later that persistent lymphadenopathy develops, progressing to suppuration in 10 to 30% of patients. This satellite adenopathy, single in 85% of cases, is accompanied by a slight hyperthermia. Lesions regress spontaneously (justifying the term “benign”) in several weeks to several months, although chronic suppuration may sometimes set in.
CSD can also manifest in various atypical forms, including Parinaud’s oculo-glandular syndrome and other sometimes serious presentations (endocarditis, encephalitis, septicemia, purpura), even in immunocompetent subjects. In immunocompromised patients, bacillary angiomatosis and peliosis represent the main clinical manifestations.
Diagnosis is based on epidemiological and clinical criteria. Differential diagnosis includes other adenopathies related to various general diseases (rubella, tularemia) or to common wounds, bites or scratches infected by non-specific bacteria or by Pasteurella spp.
Cats that are transmission vectors remain clinically healthy. The infectious agent can only be isolated in a bacteremic cat by blood culture and PCR identification. Serology is also usable, but a positive reaction is not necessarily correlated with active bacteremia.
Antibiotic therapy, even prolonged, does not appear to eliminate bacteremia in cats. Specific prophylactic measures therefore remain limited. On the other hand, regular use of flea products can reduce contamination of the feline reservoir. It should be noted that declawing, sometimes proposed as a preventive measure, is of no interest.
Prevention is based on clear information for at-risk persons (particularly immunocompromised patients), reasoned flea control in cats, hand washing after contact with the animal, and, as for all diseases transmitted by bites or scratches, washing and immediate disinfection of wounds.
Pasteurelloses
Animal pasteurelloses, frequently encountered in many species (ruminants, pigs, poultry, lagomorphs), clinically manifest by various conditions: broncho and pleuropneumonias, subcutaneous abscesses, or septicemic forms (fowl cholera).
These infections are transmitted to humans through the usual modes of contagion (direct contact, food, inhalation), but the main mechanism is inoculation by cat bite, dog bite, more rarely rat or rabbit bite. This bite can be inflicted by a clinically sick animal, but more often by an apparently healthy animal, Pasteurella spp. being a commensal bacterium of the upper aerodigestive tract of many animals, isolated in 40 to 80% of samples in the species concerned.
The pasteurellae isolated from bitten people are mainly P. multocida, P. canis and P. dagmatis. Cases of human pasteurellosis without identified bite are exceptional; they include pneumonia, pleurisy, pericarditis, endocarditis, arthritis, and septicemia. If animal-origin contamination by inhalation or ingestion is possible, Pasteurella spp. could also, in humans as in animals, survive as commensals on mucous membranes and only express their pathogenic power in association with debilitating conditions or diseases (viral infections, cancers, uremic syndrome, cirrhosis). In these particular cases, these pasteurelloses would not be strictly considered zoonoses.
In humans, clinical presentation is dominated by forms localized to the cutaneous entry point. Acute forms are characterized by intense and early local inflammatory signs. In the hours following germ penetration, the wound (often initially inapparent) becomes hot, red, edematous, and very painful; suppuration rapidly appears in the form of a few serous droplets. Lymphangitis and satellite adenopathy are frequently associated.
Subacute loco-regional forms evolve differently: after similar or more discreet initial manifestations, painful and persistent tenosynovitis, non-suppurative, appear near the inoculation point, or metacarpophalangeal arthropathies accompanied by vasomotor disorders (sensation of heaviness, cyanosis or pallor, paresthesia).
Clinical diagnosis is based on the rapid development of edematous inflammation of the bitten region. Bacteriological isolation from pus should be performed early on ordinary media, but results are variable.
Treatment of inoculation pasteurelloses involves tetracyclines. Human prophylaxis is complex due to the impossibility of eliminating the animal reservoir in permanent contact with humans. Given the frequency of feline contaminations and the functional sequelae sometimes observed, a preventive measure considered is to administer immediate antibiotic treatment to anyone bitten or scratched, even in the absence of early clinical signs.
Viral dermatozoonoses
Cowpox virosis
Cowpox virosis is a viral disease caused by an orthopoxvirus, the cowpox virus, described in many species: cow, camel, buffalo, rabbit, cat, and, more recently, rat. Viruses of this family (smallpox, cowpox, vaccinia, and monkeypox) are closely related and all belong to the genus Orthopoxvirus. These agents present difficulties in isolation, even from infected lesions and organs.
Diagnosis of Orthopoxvirus infection can be established by various techniques:
- Electron microscopy
- Serology
- Gene amplification (PCR)
- DNA sequencing after isolation or culture, for precise identification of the viral species
Given their genetic proximity, identification errors are possible between these viruses.
In cats, poxvirosis has been observed for approximately 30 years in Great Britain, the Netherlands, Belgium, and Germany. Its presence in France has been regularly reported since 1999. This infection almost exclusively affects rural hunting cats. Contamination mainly comes from small wild rodents (voles, field mice), more rarely from cattle.
The bank vole (Clethrionomys glareolus) and, to a lesser extent, the field vole (Microtus agrestis) play a predominant role in maintaining the infection. These rodents can also transmit the virus to other species sharing the same natural habitat (syntopic), such as the wood mouse (Apodemus sylvaticus), or even gerbils and ground squirrels in eastern regions. Cases have also been reported in rats imported from Eastern European countries. The seasonal increase in cases (summer and autumn) corresponds to the period of main activity and proliferation of these small rodents.
Transmission mainly occurs transcutaneously, sometimes oronasally.
Clinically, in cats, infection initially manifests as a single maculopapular and erythematous lesion, located on the head, neck, or forelimbs. In about ten days, numerous secondary pruritic lesions appear: macules, papules, erythematous nodules, which progressively ulcerate and can reach the entire body, including the oral cavity. General signs (fever, rhinitis, conjunctivitis) are frequently observed.
Evolution is generally favorable, with spontaneous healing of secondary lesions in 3 to 8 weeks. However, complications such as bacterial superinfections or retroviral co-infection can cause generalization of skin lesions and sometimes fatal pneumonia.
In rats, cutaneous manifestations are comparable to those of cats.
Transmission to humans has been documented both from cats and rats, with a particularly reserved prognosis in immunocompromised or elderly individuals. Cessation of smallpox vaccination may have reduced cross-protection against poxviruses in the general population, thus predisposing unvaccinated subjects, particularly if immunocompromised, to these infections.
In humans, after an incubation of 2 to 6 days, cutaneous manifestations of cowpox are generally benign: papular, vesicular, umbilicated, and haloed lesions, located on the face, hands, arms, and sometimes mucous membranes (especially in children). General signs (fever, adenopathy) frequently accompany the eruption. In immunocompromised patients, infection can take a severe form with generalized pustular and hemorrhagic smallpox, potentially fatal.
Diagnosis in animals is mainly based on histopathology of skin biopsies, which reveals specific poxvirus lesions. Other less common techniques include electron microscopy, serology, viral isolation, and PCR.
Treatment in cats is essentially symptomatic, aimed at controlling bacterial superinfections and maintaining adequate nutrition despite painful oral lesions.
Prophylactic measures are fundamental:
- Isolation of the sick cat to avoid inter-feline contamination
- Euthanasia of affected rats
- Disinfection of the environment (bleach) due to virus resistance
- Precautions during handling (wearing gloves) to limit zoonotic risk, particularly for vulnerable people (immunocompromised, children, elderly)
Public health implications and prevention strategies
Dermatozoonoses, although less publicized than other systemic zoonoses, represent a non-negligible public health issue, particularly in the context of an increasingly close human-animal relationship. A retrospective survey in a veterinary dermatology practice reveals that nearly 35% of owners share their bed with their pet (cat or dog), and persist in this habit even when they themselves present dermatological lesions attributable to their companion.
This proximity, associated with the multiplicity of potentially transmissible pathogens, underscores the importance of a coordinated preventive approach between physicians, veterinarians, and pet owners.
Role of the veterinary practitioner
The veterinary practitioner occupies a strategic position at the interface between animal and human health. Their role is not limited to the diagnosis and treatment of animal conditions; it extends to:
- Owner information regarding the potential zoonotic risks associated with their animal
- Education on preventive measures adapted to each situation
- Early detection of conditions with zoonotic potential
- Implementation of appropriate treatments aimed not only at healing the animal but also at interrupting the chain of transmission to humans
- Collaboration with physicians for global management of cases involving transmission to humans
This public health mission proves particularly delicate because it requires reconciling the emotional attachment of owners to their animals with sanitary imperatives. It is often illusory to want to radically modify cohabitation behaviors between owners and their companions, but clear and objective information generally allows the adoption of reasonable precautions.
Populations at particular risk
Certain populations present increased vulnerability to dermatozoonoses and deserve specific attention:
- Immunocompromised persons (patients on immunosuppressants, people living with HIV, transplant recipients, patients undergoing chemotherapy): HIV infection, in particular, confers a particular dimension to zoonotic risk, with potentially more serious manifestations of conditions such as sporotrichosis, leishmaniasis, poxviroses, or tuberculosis
- Young children: their still immature immune system, associated with risky behaviors (close contact with animals, non-systematic hand hygiene) particularly exposes them
- Elderly people: age-related immune fragility
- Pregnant women: specific risks related to certain pathogens
- Professionals in contact with animals: veterinarians and their staff, breeders, groomers, shelter staff
For these populations, specific recommendations must be formulated, ranging from simple reinforced hygiene precautions to temporary eviction of certain animal species depending on the clinical context.
Specific preventive strategies
For dermatophytoses
- Screening for asymptomatic carriers in animal communities
- Isolation and early treatment of affected animals
- Rigorous disinfection of the environment
- Precautions when acquiring new animals (particularly kittens from breeding facilities or pet stores)
- Particular awareness of those responsible for childcare communities (schools, nurseries) to the risks associated with class mascots
For scabies and other ectoparasitoses
- Regular antiparasitic treatment of pets
- Control of stray animal populations
- Increased precautions when adopting animals from shelters
- Identification and treatment of all animals in contact in case of a positive diagnosis
For leishmaniasis
- Annual screening in enzootic areas
- Use of repellents against phlebotomine sandflies during the vector activity season
- Limitation of nocturnal outings of dogs in endemic regions
- Rigorous monitoring of infected dogs
- Clear information for owners on risks and prevention measures
For diseases transmitted by bites or scratches
- Owner education on appropriate animal handling
- Immediate disinfection of any wound, even minor
- Rapid medical consultation in case of inflammatory signs
- Flea control program to limit transmission of B. henselae in cats
“One Health” Approach
The “One Health” concept recognizes the interdependence between human health, animal health, and environmental health. This approach is fully meaningful in the management of dermatozoonoses, which perfectly illustrate this interconnection.
Close collaboration between physicians and veterinarians constitutes the cornerstone of effective management of these pathologies.
This cooperation should be structured around several axes:
- Information sharing on detected cases and epidemiological developments
- Standardization of diagnostic protocols to facilitate comparisons between human and animal cases
- Coordination of therapeutic approaches to prevent the emergence of resistances
- Joint development of coherent preventive messages for the public
- Collaborative research on transmission mechanisms and risk factors
This interprofessional collaboration must be part of a broader framework also involving:
- Public health authorities
- Diagnostic laboratories
- Research structures
- Animal protection associations
- Breeding and pet store professionals
Only this integrated approach will allow optimal management of these conditions at the human-animal interface.
Fundamental precautions
Some fundamental precautions, valid for all dermatozoonoses, can be recommended to pet owners and professionals:
- Rigorous hand hygiene after any contact with animals, particularly before meals
- Regular deworming and external deparasitization of domestic animals
- Regular veterinary monitoring with explicit mention of any contact with vulnerable persons
- Frequent cleaning of animal bedding areas
- Wearing gloves when handling animals with skin lesions
- Temporary avoidance of close contact (bed sharing, face licking) in case of diagnosed animal dermatosis
- Rapid medical consultation in case of appearance of skin lesions in humans after contact with a sick animal
These simple measures, combined with increased awareness of alarm signs among owners, would significantly reduce the incidence of human transmission cases.
Conclusion
Dermatozoonoses are a heterogeneous group of skin conditions transmissible between vertebrate animals and humans, with a relatively low prevalence compared to all zoonoses, but a potentially significant impact on public health. With the exception of a few specific entities such as sporotrichosis, cat scratch disease, leishmaniasis and cowpox virosis, these conditions rarely present a character of medical severity in humans, who generally constitute a parasitic dead end.
However, the recovery of the affected owner, with or without treatment, is doomed to failure if the source of contamination – the animal – is not identified and treated adequately. This interdependence underscores the crucial importance of a coordinated approach between veterinary and human medicine.
The veterinary practitioner, by their strategic position at the human-animal interface, plays a key role in early detection, appropriate treatment, and prevention of these conditions. Their responsibility extends beyond animal care to encompass a public health dimension, involving information, education, and collaboration with physicians.
In a context of increasing integration of pets within families, with increasingly frequent close physical contact, vigilance against dermatozoonoses is gaining in importance. This proximity, although a source of undeniable psychological and social benefits, requires adequate medicalization of pets as an indispensable corollary to their family integration.
Finally, the “One Health” approach, recognizing the interconnection between human health, animal health, and environmental health, provides a relevant conceptual framework for addressing these pathologies. Only close collaboration between all stakeholders – veterinarians, physicians, pet owners, health authorities – will minimize transmission risks while preserving the benefits of the human-animal relationship.
FAQ
1. Do animals carrying zoonotic germs systematically show identifiable clinical signs?
No, many animals can be asymptomatic carriers of pathogens transmissible to humans. This is particularly the case for dermatophytoses (especially in cats), leishmaniasis (approximately 50% of infected dogs in enzootic areas are asymptomatic), or cat scratch disease (bacteremic cats generally show no clinical signs). This particularity complicates detection and justifies systematic preventive measures, particularly for at-risk populations.
2. How to clinically distinguish the different forms of animal scabies and assess their zoonotic potential?
The different animal scabies (sarcoptic, notoedric, trixacaric) present relatively similar clinical pictures in animals (erythematosquamous-crustous lesions and pruritus), but are distinguished by their preferential distribution and the animal species affected. Their zoonotic potential is variable: all can cause lesions in humans, but generally constitute “hemizoonoses” where the parasite cannot complete its life cycle in human skin. Precise parasitological diagnosis by skin scraping is essential to assess the risk of transmission and adapt preventive measures.
3. Do preventive antiparasitic treatments marketed for pets offer complete protection against parasitic dermatozoonoses?
Modern external antiparasitics, particularly those based on isoxazolines, avermectins, or milbemycins, offer excellent protection against most ectoparasites responsible for dermatozoonoses (fleas, sarcopts, notoedres, cheyletiellae). However, their effectiveness is not absolute and depends on multiple factors: treatment adherence, coverage of the parasitic spectrum, emerging resistances, individual particularities. In addition, these treatments generally do not offer protection against fungal, bacterial, or viral dermatozoonoses. A global preventive approach is therefore necessary, combining antiparasitic treatment, appropriate hygiene, and regular veterinary monitoring.
4. What to do when faced with an animal presenting skin lesions in a household with immunocompromised persons?
In this high-risk situation, several measures are necessary: immediate veterinary consultation for precise diagnosis, temporary isolation of the animal in a dedicated room until resolution of lesions, wearing gloves during necessary handling, rigorous disinfection of contact surfaces, and reinforcement of hand hygiene. Depending on the diagnosis established and the degree of immunosuppression of the person concerned, stricter measures may be considered, always in consultation between the veterinarian and the attending physician. In certain particular cases involving highly pathogenic agents such as Sporothrix schenckii in a severely immunocompromised patient, temporary separation may be necessary.
5. Does the increase in cases of dermatozoonoses observed in recent decades reflect a real emergence or simply better detection?
The apparent evolution of the incidence of dermatozoonoses probably results from a combination of factors. On the one hand, diagnostic advances and increased awareness among health professionals allow better case identification. On the other hand, several factors favor a real emergence: increase in the number of pets and intensification of physical contact, multiplication of international travels facilitating the circulation of new pathogens, growth of immunocompromised populations more vulnerable to these infections, environmental modifications affecting parasitic cycles, and emergence of resistance to antiparasitic treatments. A rigorous epidemiological approach, associating human and veterinary medicine, is necessary to precisely quantify these trends and adapt preventive strategies.
References
- guaguère é. dermatozoonoses en milieu urbain : le point de vue du dermatologue vétérinaire = dermatozoonoses in urban settings : the veterinary dermatologist’s point of view. 33522539.pdf.
- martin s, schmutz jl. les dermatozoonoses. nouv dermatol 2002 ; 21 : suppl.2 : 37-42.
- viaud s, bensignor e. les dermatozoonoses du chien et du chat. prat med chir anim comp 2008 : 43, 131-9.
- euzéby j. mycologie médicale comparée : les mycoses des animaux et leurs relations avec les mycoses de l’homme. tome i. oullins: fondation marcel merieux; 1992.
- moriello ka, de boer dj. dermatophyties in: guaguère e, prélaud p, editors. guide pratique de dermatologie féline. lyon: mérial; 2000. p. 4.1–11.
- brouta f, deschamps f, losson b, mignon b. données récentes sur la pathogénèse de l’infection de microsporum canis chez les carnivores domestiques. ann med vet 2001; 145: 236–42.
- mignon b. dermatophyties. in: guaguère e, prélaud p, editors. guide pratique de dermatologie canine. lyon: kalianxis; 2006. p. 153–66.
- guaguère e, hubert t, muller a. dermatologie des petits mammifères de compagnie. in : encyclopédie vétérinaire – dermatologie, elsevier, paris, 2007 ; vol 2 (3700) 1-17.
- carlotti dn. le traitement des dermatophytoses du chien et du chat. gestion de la teigne en chatterie. prat med chir anim comp 2008; 43 : 1–13.
- viguié-vallanet c. les teignes. ann dermatol venereol 1999 ; 126 : 349-56.
- ferrer l, fondati a. mycoses profondes. in: guaguère e, prélaud p, editors. guide pratique de dermatologie feline. lyon: mérial; 2000. p. 5.1-11.
- schubach tm, schubach a, okamoto t, barros mb, figueiredo fb, cuzzi t, et al. evaluation of an epidemic sporotrichosis in cats: 347 cases (1998—2001). j am vet med assoc 2004; 224: 1623-9.
- cafarchia c, sasanelli m, lia rp, de caprariis d, guillot j, otranto d. lymphocutaneous and nasal sporotrichosis in a dog from sou-thern italy: case report. mycopathologia 2007; 163: 75—9.
- verde m. dermatozoonoses. in: guaguère e, prélaud p, editors. guide pratique de dermatologie féline. lyon: mérial; 2000. p. 25.1–7.
- guaguère e, beugnet f. dermatoses parasitaires. in: guaguère e, prélaud p, editors. guide pratique de dermatologie canine. lyon: kalianxis; 2006; p 181- 231.
- paterson s. skin diseases of exotic pets (paterson s, ed), blackwell publishing, oxford, 2006 ; 333 p.
- ellis c, mori m. skin diseases of rodents and small exotic mammals. vet clin north am exotic anim pract 2007 ; 4 : 523-7.
Podcast
Search terms
zoonotic skin diseases, zoonotic diseases, farm animals, bacterial infections, infectious diseases, infected animals, zoonotic infections, serious illness, animal bites, eating contaminated food, wild animals, contaminated food, mad cow disease, viral disease, bacterial zoonoses, other zoonotic diseases, lyme disease, weakened immune systems, human health, parasitic infections, fungal diseases, animals to humans, abdominal pain, severe illness, prevent infection, parasitic zoonoses, viral zoonoses, rabies virus, q fever, immunocompromised patients, infected cat, skin lesions, lymph nodes, direct or indirect contact, wild birds, human infection, direct contact, body fluids, soil contaminated, other animals, farm workers, poor appetite, proper hygiene, indirect contact, cutaneous larva, body aches, unpasteurized milk, common symptoms, specific animals, public health, rare cases, severe cases, various organs, single celled organisms, domestic animals, disease, humans, animals, infection, drinking water, high fever, skin diseases, high mortality, illness, parasites, wearing gloves, infected, open wounds, fever, avoid contact, healthcare provider, contaminated water, symptoms, rabies, spread