Canine vasculitis represents a complex inflammatory reaction pattern targeting blood vessel walls through aberrant immune responses, manifesting from subtle cutaneous lesions to severe systemic involvement.
July 2025
Introduction to Canine Vasculitis
This comprehensive analysis provides veterinary professionals with essential insights into pathophysiology, classification systems, and evidence-based therapeutic approaches for optimal clinical outcomes.
Canine vasculitis is not a singular diagnosis but rather a complex inflammatory reaction pattern characterized by an aberrant immune response directed against the walls of blood vessels. This inflammation compromises the structural and functional integrity of arteries, veins, and capillaries. The assault on vascular structures, particularly the endothelial cells, can precipitate a cascade of deleterious events, including vessel wall leakage leading to edema and hemorrhage, luminal narrowing or occlusion from thrombosis, and subsequent ischemic injury to the affected tissue. The clinical manifestations are therefore a direct consequence of this impaired blood flow.
The fundamental pathophysiology is most often attributed to a type III hypersensitivity reaction, a process involving the formation and deposition of circulating antigen-antibody complexes, or immune complexes, within vessel walls. These complexes activate the complement cascade, which in turn recruits inflammatory cells that release destructive enzymes and reactive oxygen species, causing fibrinoid necrosis and vascular damage. While this mechanism is central, other immunologic mechanisms, such as Type II hypersensitivity reactions involving antibodies directly targeting vascular components, may also contribute to the pathogenesis.
A critical distinction in the clinical approach is between primary (or idiopathic) vasculitis and secondary vasculitis. Primary vasculitis is diagnosed when no identifiable underlying trigger can be found, a scenario that accounts for a substantial number of cases—over 50% in some retrospective analyses. Secondary vasculitis, conversely, occurs as a pathologic response to a known inciting factor, such as infectious diseases, an adverse drug reaction, or neoplasia. The skin is a frequent and highly visible target, making cutaneous vasculitis a common presentation. However, the skin can also serve as a sentinel for systemic vasculitis, a more pernicious form where multiple organ systems are involved. In these cases, cutaneous lesions may be the first and most accessible clue to a life-threatening systemic process, underscoring the importance of a thorough diagnostic evaluation.
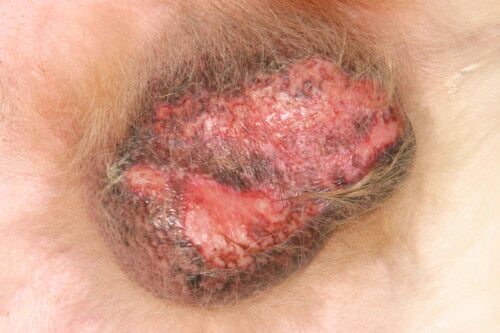
Severe testicular canine vasculitis
Types of Canine Vasculitis
The classification of canine vasculitis relies on two complementary frameworks: histopathological patterns and clinical syndromes. Histopathology, which examines the nature of the cellular infiltrate within and around vessel walls, provides the most fundamental classification. The dominant type of inflammatory cells defines the subtype, though the clinical utility of this classification for predicting etiology or therapeutic response is often limited.
Neutrophilic vasculitis is one of the most frequently identified patterns. It is characterized by a dense infiltrate of neutrophils. When these neutrophils undergo fragmentation, leaving behind nuclear dust (karyorrhexis), the condition is termed leukocytoclastic vasculitis. This pattern is often associated with infectious triggers, drug reactions, or sepsis. A lymphocytic or mononuclear pattern, dominated by lymphocytes and plasma cells, is classically associated with rabies vaccine-induced reactions. Finally, cell poor vasculitis represents a diagnostic challenge; it is characterized by evidence of vascular damage, such as fibrinoid necrosis or thrombosis, but with a conspicuously sparse inflammatory infiltrate. This form is often linked to ischemic dermatopathies where the inciting inflammatory event may have been transient.
Clinically, vasculitis is broadly categorized by its scope and etiology. Cutaneous vasculitis, where inflammation is confined to the vasculature of the skin and subcutis, is the most common presentation. In contrast, systemic vasculitis involves the blood vessels of multiple internal organs, such as the kidneys, central nervous system, or heart, and carries a more guarded prognosis. Familial vasculitis refers to specific, heritable syndromes recognized in certain breeds, the most well-documented being familial cutaneous vasculopathy in German Shepherd Dogs. When a comprehensive diagnostic investigation fails to uncover an underlying trigger in a patient with confirmed cutaneous inflammation of the vessels, a diagnosis of idiopathic cutaneous vasculitis is made. This diagnosis of exclusion represents a significant portion of cases and often necessitates long-term, empirical immunomodulatory therapy. While histopathological classification is essential for confirming a diagnosis of vasculitis, it is the clinical context and search for an underlying trigger that ultimately guides the therapeutic strategy.
Clinical Signs of Canine Vasculitis
The clinical signs of canine cutaneous vasculitis are notoriously variable, reflecting the size, location, and number of blood vessels affected, as well as the severity of the inflammatory insult. The primary lesions are a direct manifestation of vascular damage and compromised perfusion. Early or mild cases may present with subtle signs such as alopecia, erythema, or pitting edema. Urticarial plaques or wheals that persist longer than 24-48 hours are also suggestive.
As vascular injury progresses, more specific and telling lesions develop. Palpable purpura—raised, reddish-purple spots resulting from hemorrhage that do not blanch with pressure (diascopy)—is a classic sign. These may coalesce into larger ecchymotic patches or form hemorrhagic bullae. With significant ischemia, the skin lesions evolve into sharply demarcated, “punched-out” ulcers or deep, black, leathery eschars, which represent full-thickness necrosis of the skin. These ischemic cutaneous lesions are often painful.
The distribution of lesions is a key diagnostic clue. Typically, lesions occur on body regions with a tenuous or terminal blood supply. These sites include the tips of the ear pinnae, the central aspect of the paw pads, the tail tip, the nasal planum, the lips, the scrotum, and the skin overlying bony prominences such as the elbows and hocks. This classic acral distribution is a direct result of the vascular anatomy, as these areas lack robust collateral circulation and are thus exquisitely vulnerable to ischemic injury.
Many patients, particularly those with systemic vasculitis or severe acute cutaneous disease, exhibit constitutional signs. These commonly include fever, lethargy, anorexia, and demonstrable pain. Lameness may indicate involvement of joints or muscle, and significant muscle atrophy can develop secondary to myositis or disuse. In the most severe cases, vasculitis can precipitate life-threatening complications. These include disseminated intravascular coagulation (DIC), hemorrhagic uremic syndrome, and organ failure. A particularly devastating form is cutaneous and renal glomerular vasculopathy (CRGV), which manifests with skin ulceration and rapidly progressing acute renal failure.
Causes of Canine Vasculitis
The etiology of canine vasculitis is diverse, yet in a substantial number of cases—often reported as over 50%—an exhaustive search for a cause is unrewarding, leading to a diagnosis of idiopathic disease. When a trigger can be identified, the condition is classified as secondary vasculitis. A meticulous history and diagnostic workup are paramount, as identifying and removing the inciting cause is the cornerstone of successful management.
Infectious diseases are a major category of triggers. The vasculitis triggered by pathogens can result from direct invasion of endothelial cells or, more commonly, from systemic immune complex formation. A wide range of organisms have been implicated, including bacteria (e.g., Staphylococcus spp., tick-borne agents like Rickettsia, Ehrlichia, and Borrelia spp.), viruses (e.g., canine distemper virus, dog circovirus), protozoa (e.g., Leishmania spp.), and fungi (e.g., Blastomyces spp.). An early hypothesis, stemming from the first cases observed in Greyhounds in the United States, suggested a link to a verotoxin produced by E. coli. However, this association was never confirmed, and investigations into more recent and widespread outbreaks in the UK have failed to identify Shiga toxin in affected dogs. Consequently, the etiology of CRGV currently remains unknown, although active research continues into potential environmental triggers and genetic susceptibility factors.
An adverse drug reaction is another frequent cause. Numerous medications can provoke vasculitis, making a detailed drug and supplement history essential. Implicated drugs include various antibiotics (especially sulfonamides), antifungals (itraconazole), ivermectin, and even parenterally administered substances like human serum albumin. Vaccinations are also a well-recognized trigger. Post-vaccinal vasculitis, classically associated with the rabies vaccine, can manifest as localized alopecia, inflammation, or panniculitis at the injection site, or as distant skin lesions on the ear tips and extremities.
Other potential triggers include cutaneous adverse food reactions, which can present as an urticarial vasculitis. Specific food proteins, such as beef, have been implicated in some reports. Furthermore, vasculitis can be a paraneoplastic phenomenon, occurring secondary to an underlying malignancy such as lymphosarcoma. Finally, vasculitis is a cardinal feature of several primary autoimmune diseases and connective tissue diseases, which represent a distinct and important category of causes.
Autoimmune Diseases and Canine Vasculitis
Autoimmune diseases and other immune mediated diseases represent a fundamental cause of vasculitis, where the pathology arises from a failure of self-tolerance. In these conditions, an aberrant immune response is mounted against the body’s own vascular structures, leading to inflammation and damage. The blood vessels effectively become the target of a misdirected immune attack.
Several specific autoimmune conditions are strongly associated with vasculitis. Systemic lupus erythematosus (SLE) is a quintessential multi-systemic autoimmune disease in which vasculitis is a frequent and major contributor to pathology in the skin, kidneys, joints, and other organs. Its cutaneous counterpart, discoid lupus erythematosus (DLE), is primarily an interface dermatitis but can feature a significant vasculopathic component, particularly in lesions on the nasal planum.
The predominant pathogenic mechanism in these diseases is believed to be a type III hypersensitivity reaction. This process is initiated by the formation of soluble antigen-antibody complexes (immune complexes) in the circulation. These complexes deposit in the walls of small blood vessels, particularly at sites of turbulence or high pressure. This immune complex formation and deposition triggers the activation of the complement system, leading to the release of chemotactic factors that attract neutrophils. The recruited neutrophils then release lysosomal enzymes and reactive oxygen species, causing fibrinoid necrosis of the vessel wall and surrounding tissue damage. In this model, the vessel wall is often described as an “innocent bystander,” caught in the crossfire of an immune response directed against a separate antigen. While Type III hypersensitivity reactions are central, other mechanisms, such as Type II hypersensitivity involving cytotoxic antibodies directed against endothelial cells, may also play a role.
Other immune mediated conditions can also be associated with vasculitis. For example, chronic inflammation associated with atopic dermatitis may, in some cases, predispose to or coexist with vasculitic changes. Cold agglutinin disease, an immune mediated hemolytic anemia, can lead to ischemic vasculopathy in acral sites due to temperature-dependent red blood cell agglutination and occlusion of small vessels.
Familial and Genetic Factors
A genetic predisposition for developing canine vasculitis is strongly suspected in several breeds, which are consistently overrepresented in clinical reports. These breeds include the German Shepherd Dog, Jack Russell Terrier, Greyhound, Scottish Terrier, Saint Bernard, and Chinese Shar-Pei. The existence of distinct, breed-specific syndromes provides compelling evidence for the role of heritable factors in the pathogenesis of this vascular disease.
The most well-characterized of these conditions is familial cutaneous vasculopathy in German Shepherd Dogs. This syndrome typically manifests in young puppies, often between 6 and 8 weeks of age, with the onset of clinical signs frequently coinciding with the first puppy vaccinations. This temporal association suggests that the immune stimulation from vaccination acts as a trigger for a latent genetic defect. Affected puppies present with systemic signs of illness, including fever and lethargy, along with characteristic cutaneous lesions: swelling and crusting of the nasal bridge, ulceration of the ear pinnae and tail tip, and markedly swollen, painful, and depigmented footpads. While many puppies recover, relapses can occur with subsequent vaccinations. The mode of inheritance is hypothesized to be autosomal recessive.
Other breeds exhibit unique familial vasculitis syndromes. Greyhounds are susceptible to a severe condition known as cutaneous and renal glomerular vasculopathy (CRGV), or “Alabama Rot,” which causes ulcerative skin lesions on the limbs and is often complicated by fatal acute renal failure. Jack Russell Terriers and Scottish Terriers have their own reported forms of familial disease. The Saint Bernard is the breed most commonly affected by proliferative arteritis of the nasal philtrum, a specific dermal arteritis where a familial link is strongly suspected due to the close relation of many reported cases.
|
Syndrome Name |
Associated Breeds |
Typical Age of Onset |
Key Clinical Features |
Suspected Inheritance / Trigger |
|
Familial Cutaneous Vasculopathy |
German Shepherd Dog |
6-8 weeks |
Fever, lethargy; swollen, crusted nose; ulcerated ear/tail tips; swollen, depigmented footpads |
Autosomal recessive; triggered by vaccination |
|
Cutaneous and Renal Glomerular Vasculopathy (CRGV) |
Greyhound (and other breeds) |
Young adult |
Ulcerative skin lesions on limbs/ventrum; pitting edema; rapidly progressive acute renal failure |
Unknown; E. coli verotoxin proposed |
|
Proliferative Arteritis of the Nasal Philtrum |
Saint Bernard, Giant Schnauzer |
Adult (3-6 years) |
Linear ulcer on the nasal philtrum; risk of severe arterial hemorrhage |
Familial predisposition suspected in Saint Bernards |
|
Familial Vasculitis |
Jack Russell Terrier |
Variable |
Classic acral distribution of lesions (ear tips, paw pads, tail tip) |
Genetic predisposition |
|
Hereditary Nasal Arteritis |
Scottish Terrier |
3-4 weeks |
Progressive ulceration and destruction of the nasal planum and nares |
Probable genodermatosis |
Clinical Findings and Diagnosis
The diagnosis of canine vasculitis is a deductive process that integrates historical information, physical examination findings, laboratory testing, and ultimately, histopathology. A definitive diagnosis requires a high index of suspicion and a systematic approach to rule out mimickers and identify any underlying disease.
The initial clinical findings are paramount. A thorough history must be obtained, focusing on potential triggers such as recent medications, vaccinations, dietary changes, or travel to areas endemic for infectious diseases. The physical examination should carefully characterize the morphology and distribution of skin lesions. The presence of “punched-out” ulcers on paw pads, necrotic ear tips, or palpable purpura should immediately elevate vasculitis on the list of differentials. Diascopy, the technique of pressing a glass slide against an erythematous lesion, is a simple and useful test; failure of the lesion to blanch confirms hemorrhage (purpura).
A baseline laboratory evaluation is essential. This includes a complete blood count (CBC), a serum biochemistry profile, and a urinalysis. While often non-specific, these tests can provide evidence of systemic inflammation or organ involvement. The CBC may reveal a neutrophilia, non-regenerative anemia, or thrombocytopenia. The biochemistry profile might show elevated liver enzymes or azotemia, indicating hepatic or renal compromise, respectively. Hypoalbuminemia can result from protein-losing nephropathy or enteropathy, or as a negative acute-phase reactant. Urinalysis is critical for detecting proteinuria, which can be an early sign of glomerular damage.
Given the role of infections as a trigger, screening for relevant pathogens is a crucial step, particularly before initiating immunosuppressive therapy. This may involve serology or PCR for tick-borne diseases and other regional infectious agents.
The gold standard for a definitive diagnosis is histopathological examination of skin biopsies. The success of this procedure hinges on proper site selection and technique. Multiple (3-4) deep punch or wedge biopsies should be harvested from the active margins of the newest, most representative, non-ulcerated lesions, such as erythematous plaques or purpuric macules. Submitting biopsies from the center of chronic ulcers is often unrewarding, yielding only non-specific evidence of necrosis and secondary infection. The pathologist will look for the hallmark features of vasculitis: inflammation centered on and infiltrating vessel walls, fibrinoid degeneration, thrombosis, hemorrhage, and secondary ischemic changes to the surrounding dermis and epidermis. The type of inflammatory infiltrate (neutrophilic vasculitis, lymphocytic, etc.) should also be reported. It is important to recognize that the lesions of vasculitis can be transient and segmental; therefore, a negative or non-diagnostic biopsy does not entirely rule out the disease if clinical suspicion remains high.
While most cases of vasculitis in dogs present with classic cutaneous signs, there are several rare and distinctive forms that warrant special attention. Proliferative thrombovascular necrosis is a severe condition characterized by progressive necrosis of the skin due to extensive thrombosis and inflammation within the blood vessels. This form can lead to significant tissue loss and may require aggressive intervention.
Another unusual presentation is multifocal erythematous cutaneous swellings, where dogs develop multiple, raised, red swellings on the skin, often accompanied by systemic signs. Eosinophilic vasculitis is a rare subtype marked by a predominance of eosinophils in the inflammatory infiltrate, and may be associated with hypersensitivity reactions or parasitic infections.
Breed-specific syndromes also exist, such as familial cutaneous vasculopathy in German Shepherds, which often manifests in young puppies and may be triggered by vaccinations or other immune stimuli. Idiopathic cutaneous and renal glomerular vasculopathy is a particularly serious and rare condition that affects both the skin and kidneys, leading to cutaneous and renal glomerular involvement and potentially life-threatening complications.
Proliferative arteritis of the nasal philtrum is another rare entity, most commonly seen in Saint Bernards, where inflammation and thickening of the arteries in the nasal philtrum result in sharply demarcated ulcers and a risk of severe hemorrhage. Early recognition and specialized treatment, which may include surgical intervention, are crucial for these rare forms. Prompt diagnosis and tailored therapy can help prevent long-term damage and improve outcomes, even in these challenging cases.
Dermal Arteritis and Canine Vasculitis
Dermal arteritis represents a specific and often severe form of canine vasculitis in which the inflammatory process primarily targets the dermal arteries and arterioles. These are medium-sized vessels, larger than the post-capillary venules typically affected in more common forms of small-vessel vasculitis. The involvement of arteries, which supply significant territories of skin, means that inflammation, mural thickening (proliferative arteritis), and subsequent occlusion can lead to extensive and deep ischemic necrosis.
This category encompasses several distinct clinical syndromes that are recognized by their unique presentations and histopathological features. These include proliferative thrombovascular necrosis of the pinnae, a condition causing progressive ischemic necrosis of the ear flap, and the particularly notable entity known as arteritis of the nasal philtrum. What unifies these conditions is the pathological finding of an arteritis or arteriopathy, distinguishing them from the more common venulitis or capillaritis. The diagnosis often relies on deep biopsies that can capture these larger vessels in the deep dermis or subcutis. Management must be aggressive to counteract the profound reduction in blood flow and prevent irreversible tissue loss.
Arteritis of the Nasal Philtrum
Arteritis of the nasal philtrum, also known as proliferative arteritis of the nasal philtrum, is a rare and highly distinctive vascular disease. It is a form of dermal arteritis that selectively affects the medium-sized dermal arteries located within the nasal philtrum, the vertical groove separating the nostrils on the nasal planum. The condition is characterized by a pathognomonic clinical lesion: a solitary, well-demarcated, linear or V-shaped ulcer that develops directly over the philtrum.
The underlying pathology involves a profound inflammation and proliferation of spindle cells within the arterial walls, leading to significant thickening, luminal stenosis, and thrombosis. This vascular occlusion results in ischemic necrosis of the overlying skin and the formation of the characteristic ulcer. While the ulcer itself is a significant lesion, the most critical clinical concern is the potential for profuse, pulsatile arterial hemorrhage. Erosion of the weakened arterial wall can lead to dramatic bleeding that constitutes a medical emergency.
There is a marked breed predisposition, with Saint Bernards being significantly overrepresented in the literature. Giant Schnauzers and other large-breed dogs have also been reported. The clustering of cases within related Saint Bernard lines strongly suggests an inherited or familial basis for the disease.
Diagnosis is often made presumptively based on the unique and unmistakable clinical presentation. Biopsy can confirm the underlying arteritis but may be deferred if the risk of hemorrhage is high. Treatment is multimodal, aiming to control the inflammation and prevent bleeding. Medical management includes potent topical therapies like tacrolimus ointment, systemic corticosteroids (prednisone), and other immunomodulatory agents such as the combination of doxycycline and niacinamide, or pentoxifylline. In cases with severe or recurrent hemorrhage, surgical intervention to ligate the affected artery or excise the lesion may be required.
Cutaneous Vasculitis
Cutaneous vasculitis is the most common clinical manifestation of the vasculitic reaction pattern in dogs. It is defined by inflammation that is confined to the blood vessels of the skin and subcutis. The clinical presentation of canine cutaneous vasculitis is a spectrum, ranging from relatively mild skin lesions such as persistent urticarial plaques, purpura, and edema, to severe, full-thickness ischemic injury resulting in painful, “punched-out” ulcers and necrotic eschars.
The primary diagnostic and therapeutic challenge associated with cutaneous vasculitis in dogs is the frequent inability to identify an inciting cause. After a thorough diagnostic workup has failed to reveal an underlying disease, drug, or other trigger, a diagnosis of idiopathic cutaneous vasculitis is made. This is a diagnosis of exclusion that necessitates a comprehensive investigation to rule out all known causes of secondary vasculitis.
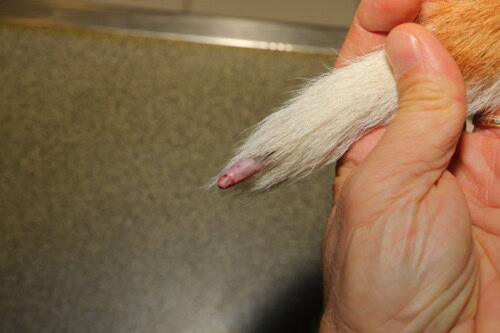
Caudal canine vasculitis

Auricular canine vasculitis
Management of cutaneous vasculitis is guided by two core principles. The first and most critical is the identification and elimination of any identifiable trigger. The second, which is the cornerstone of therapy for idiopathic cases, is the suppression of the aberrant immune response using immunomodulatory or immunosuppressive medications. The intensity of therapy is tailored to the severity of the disease. Milder, non-ulcerative cases may be managed with agents like pentoxifylline or the combination of doxycycline and niacinamide. However, severe, rapidly progressive, or ulcerative disease typically requires more potent immunosuppression with drugs like corticosteroids to halt the inflammatory cascade and prevent further tissue damage. A crucial component of management is supportive care for ulcerated lesions, including gentle cleansing and systemic antibiotics to control or prevent secondary bacterial infections, which can significantly complicate healing.
Treatment and Management of Canine Vasculitis
The management of canine vasculitis is a nuanced process that must be individualized based on the severity of disease, the presence of systemic involvement, and whether an underlying cause has been identified. The therapeutic strategy is typically tiered, aiming to achieve clinical remission while minimizing adverse effects.
The first and most important step is to identify and eliminate any potential triggers. In cases of secondary vasculitis, addressing the underlying disease—be it treating infectious diseases, discontinuing an offending drug, or instituting an elimination diet for cutaneous adverse food reactions—is the most direct path to resolution.
Supportive care is critical for patients with severe clinical signs. Dogs with extensive ulceration require meticulous wound management to prevent secondary bacterial infections and promote healing. This may involve gentle cleansing, topical antibacterial agents, and systemic antibiotics. Patients with systemic illness may require hospitalization for intravenous fluid therapy, nutritional support, and pain management.
Pharmacotherapy is the cornerstone of treatment for idiopathic cutaneous vasculitis and is used as an adjunct in severe secondary cases. The choice of agent depends on disease severity. For severe, acute, or ulcerative disease, systemic glucocorticoids are the mainstay for rapid suppression of inflammation. For milder or more chronic cases, or as steroid-sparing agents, several other drugs are employed. Pentoxifylline improves microcirculatory blood flow and has immunomodulatory effects. The combination of doxycycline and niacinamide offers anti-inflammatory benefits with a favorable safety profile. For refractory disease or when glucocorticoid side effects are prohibitive, more potent immunosuppressants such as cyclosporine, azathioprine, or mycophenolate mofetil are used. Often, multiple medications are used in combination to target different aspects of the inflammatory cascade and allow for lower doses of each individual drug. Topical therapy with potent corticosteroids or tacrolimus is highly valuable for managing focal lesions and can significantly reduce the need for systemic treatment.
Table 2: Pharmacotherapy for Canine Vasculitis
|
Drug Class |
Drug Name |
Mechanism of Action |
Recommended Dosage (mg/kg) |
Indication/Tier |
Key Adverse Effects/Monitoring |
|
Glucocorticoids |
Prednisone / Prednisolone |
Broad anti-inflammatory and immunosuppressive effects |
1-2 mg/kg PO SID-BID, then taper slowly |
Severe/acute disease; initial control |
Polyuria, polydipsia, polyphagia, muscle wasting, iatrogenic Cushing’s |
|
Rheologic Agents |
Pentoxifylline |
Improves RBC deformability; inhibits TNF-α and other cytokines |
15-25 mg/kg PO BID-TID |
Mild disease; steroid-sparing adjunct |
GI upset (vomiting, anorexia); generally well-tolerated |
|
Tetracycline/Niacinamide |
Doxycycline + Niacinamide |
Anti-inflammatory and immunomodulatory effects |
Doxycycline: 5 mg/kg PO BID. Niacinamide: 250-500 mg/dog PO BID-TID |
Mild immune mediated disease; steroid-sparing |
GI upset (usually from niacinamide); give with food |
|
Calcineurin Inhibitors |
Cyclosporine (modified) |
Inhibits T-cell activation and cytokine production |
5 mg/kg PO SID |
Moderate-severe disease; steroid-sparing |
GI upset, gingival hyperplasia, hypertrichosis; monitor blood levels |
|
Other Immunosuppressants |
Azathioprine |
Purine analog, inhibits lymphocyte proliferation |
1-2 mg/kg PO SID or EOD (dogs only) |
Severe/refractory disease; steroid-sparing |
Myelosuppression, hepatotoxicity; requires CBC/biochem monitoring |
|
Other Immunosuppressants |
Mycophenolate Mofetil |
Inhibits purine synthesis in lymphocytes |
10-20 mg/kg PO BID |
Severe/refractory disease; steroid-sparing |
GI upset (diarrhea); generally safer than azathioprine |
Prevention and Avoidance
Prevention of vasculitis in dogs is primarily focused on avoiding known triggers and managing the disease long-term to prevent relapses, rather than preventing its initial onset. For the large subset of dogs with idiopathic disease, primary prevention is not possible. Therefore, the strategy shifts to secondary prevention: minimizing the frequency and severity of flare-ups after a diagnosis has been established.
Long-term management is the reality for many dogs, as cutaneous vasculitis often follows a chronic, relapsing course that requires lifelong therapy to maintain clinical remission. The cornerstone of prevention is the strict avoidance of any identified triggers. If an adverse drug reaction was the cause, that specific medication and any chemically related drugs must be permanently avoided. For vaccine-reactive dogs, a careful risk-benefit discussion is warranted. This may involve measuring vaccine titers to assess existing immunity, forgoing non-core vaccinations, and separating essential vaccines in time to minimize immune stimulation. If a food allergy is confirmed, a strict avoidance diet is curative and preventative.
For dogs with secondary vasculitis, diligent management of the underlying disease is critical. For example, controlling autoimmune diseases like systemic lupus erythematosus with appropriate maintenance therapy will, in turn, control the associated vasculitis.
Most dogs with idiopathic disease will require continuous, low-dose maintenance medication. The therapeutic goal is to find the lowest effective dose of the safest possible drug or combination of drugs that prevents recurrence of skin lesions. This often involves a slow and careful tapering process over many months. Regular follow-up examinations with a veterinarian are essential to monitor for early signs of a flare-up, which allows for prompt adjustment of the treatment plan, and to screen for potential long-term side effects of the medications being used.
Prognosis and Outcome
The prognosis for dogs diagnosed with canine cutaneous vasculitis is highly variable and depends on several key factors, including the underlying cause, the extent and severity of the disease, and the timeliness of intervention. Dogs with mild forms of cutaneous vasculitis—where lesions are limited to the skin and there is no evidence of systemic involvement—often respond well to treatment and can achieve long-term clinical remission. Early recognition and prompt initiation of therapy are critical in these cases, as they help prevent progression to more severe tissue damage.
However, the outlook becomes more guarded in cases where systemic vasculitis is present, or when the disease affects vital organs such as the kidneys. Dogs that develop renal glomerular vasculopathy or the more severe cutaneous and renal glomerular vasculopathy (CRGV) face a higher risk of complications, including acute kidney injury and multi-organ dysfunction, which can significantly worsen the prognosis. In these cases, aggressive management and close monitoring are essential, but even with optimal care, some dogs may experience irreversible organ damage or succumb to their illness.
Regular follow-up visits and ongoing assessment are vital for all dogs with canine cutaneous vasculitis. This allows for timely adjustments to the treatment plan, early detection of relapses, and management of any medication side effects. With diligent care, many affected dogs can enjoy a good quality of life, and a significant proportion will achieve and maintain clinical remission.
Living with Canine Vasculitis
Managing a dog with canine vasculitis is a long-term commitment that requires vigilance, adaptability, and close collaboration with your veterinary team. Owners should become familiar with the clinical signs of vasculitis, such as the sudden appearance of skin lesions, including purpura, ulcers, or areas of necrosis, and report any changes promptly. Early intervention can prevent minor issues from escalating into more serious complications.
Routine veterinary check-ups are essential, as they allow for regular monitoring of the dog’s overall health, assessment of treatment efficacy, and early detection of potential side effects from medications. Blood tests and urinalysis may be recommended periodically to monitor organ function, especially in dogs receiving immunosuppressive therapy.
It is also important to be aware of potential cutaneous adverse food reactions or adverse drug reactions, as these can trigger or worsen vasculitis. Owners should keep a detailed record of all medications, supplements, and dietary changes, and communicate any concerns to their veterinarian. In some cases, dietary trials or medication adjustments may be necessary to identify and eliminate triggers.
Providing a stable, low-stress environment, ensuring regular gentle exercise, and maintaining a balanced, veterinarian-approved diet can all contribute to a dog’s well-being. With attentive care and a proactive approach, many dogs with vasculitis can lead happy, active lives despite their diagnosis.
Current Research and Developments in Canine Vasculitis
Ongoing research into canine vasculitis is shedding light on the complex interplay of genetic, immunologic, and environmental factors that drive this group of diseases. Investigators are particularly interested in the role of immune mediated diseases such as systemic lupus erythematosus and discoid lupus erythematosus, which are known to trigger vasculitic processes through mechanisms like type III hypersensitivity reaction and immune complex deposition.
Recent studies are also exploring the impact of hypersensitivity reactions—including those to drugs and foods—on the development of vasculitis. The identification of cutaneous adverse food reactions and adverse drug reactions as potential triggers has led to increased emphasis on detailed patient histories and elimination trials in the diagnostic workup.
Advances in treatment are another area of active investigation. The use of immunosuppressive agents such as glucocorticoids, azathioprine, and newer therapies is being evaluated for their efficacy and safety in managing both idiopathic and secondary forms of vasculitis. Researchers are also examining the role of infectious diseases as inciting factors, aiming to better understand how pathogens may initiate or exacerbate the vasculitic process.
As our understanding of the underlying immune mediated mechanisms grows, there is hope for the development of more targeted therapies and improved diagnostic tools. Continued research is essential to unravel the complexities of canine vasculitis and to provide veterinarians with evidence-based strategies for diagnosis, management, and long-term care.
Conclusion
Canine vasculitis emerges not as a single disease entity, but as a complex immunopathological reaction pattern with diverse etiologies and clinical expressions. Its presentation ranges from subtle cutaneous lesions to severe, life-threatening systemic vasculitis. While the clinical picture can be varied, the presence of characteristic ischemic lesions in acral locations—such as “punched-out” ulcers on paw pads and necrosis of the ear tips—serves as a crucial diagnostic clue for the clinician. Definitive diagnosis hinges on histopathological confirmation from carefully selected skin biopsies, though a non-diagnostic result in the face of strong clinical evidence should not preclude a presumptive diagnosis. The high prevalence of idiopathic cases underscores the challenge of management, which must follow a dual path: a diligent and persistent search for and elimination of underlying triggers, coupled with a tailored, often lifelong, immunomodulatory therapeutic plan designed to control the aberrant immune response.
The current understanding of canine vasculitis, while advanced, leaves several critical areas ripe for investigation. Future research should be prioritized in the following domains to advance diagnostic and therapeutic capabilities:
- Genomic and Genetic Investigations: Large-scale, multi-breed genome-wide association studies (GWAS) are needed to identify specific risk loci and causative mutations for familial vasculitis syndromes and to explore the genetic basis of idiopathic disease. Success in this area could lead to the development of genetic screening tests for at-risk breeds, inform breeding decisions, and provide profound new understanding of the underlying immunologic mechanisms.
- Standardization of Therapeutic Protocols: There is a pressing need for prospective, randomized, controlled clinical trials to establish evidence-based treatment guidelines. Such studies should compare the efficacy, safety, and long-term outcomes of different therapeutic strategies for idiopathic cutaneous vasculitis, for instance, evaluating prednisone versus cyclosporine as first-line agents for severe disease or assessing the true steroid-sparing effect of agents like pentoxifylline and doxycycline/niacinamide.
- Biomarker Discovery and Validation: Research aimed at identifying and validating non-invasive biomarkers is essential. The discovery of specific serum cytokines, anti-endothelial cell antibody profiles, or other circulating factors that correlate with disease activity could revolutionize the field. Such biomarkers could facilitate earlier diagnosis, provide prognostic information, and allow for objective monitoring of therapeutic response, thereby reducing the reliance on repeated, invasive skin biopsies.
- Investigation of the Microbiome’s Role: The influence of the cutaneous and gastrointestinal microbiome on immune homeostasis is an exciting frontier. Studies exploring whether dysbiosis contributes to the development or exacerbation of vasculitis triggered by an aberrant immune response could unveil entirely new pathogenic pathways and open the door to novel therapeutic interventions, such as targeted probiotics or fecal microbiota transplantation.
Références
- Favrot, C., Olivry, T., & Dunston, S. M. (2015). Histologic and clinical features of primary and secondary vasculitis: a retrospective study of 42 dogs (2004-2011). Journal of Veterinary Diagnostic Investigation, 27(4), 489–496.
- Khorzad, R., Whelan, M., Sisson, A., & Bédard, C. (2014). Cholesterol embolism syndrome in a dog with vasculitis and hypercholesterolemia. The Canadian Veterinary Journal, 55(4), 359–363.
- Reineke, E. L., Rees, C. A., Drobatz, K. J., & Clarke, D. L. (2023). Etiology, organ system involvement, and outcome of dogs with peripheral edema: 520 cases (2013-2018). Journal of Veterinary Internal Medicine, 37(5), 1774–1783.
- Nuttall, T., & Cole, L. K. (2022). Evidence-based veterinary dermatology: a systematic review of interventions for canine otitis externa. Veterinary Dermatology, 33(3), 191-e48.
- Li, L., Pesavento, P. A., Woods, L. W., & Delwart, E. (2013). A novel circovirus in dogs with hemorrhagic gastroenteritis and vasculitis. Emerging Infectious Diseases, 19(4), 534–541.
- Innera, M. (2001). A retrospective study of canine and feline cutaneous vasculitis. Veterinary Dermatology, 12(5), 255–264.
- Littman, M. P., Gerber, B., Goldstein, R. E., Labato, M. A., Lappin, M. R., & Moore, G. E. (2018). ACVIM consensus update on Lyme borreliosis in dogs and cats. Journal of Veterinary Internal Medicine, 32(3), 887–903.
- Solano-Gallego, L., Miró, G., Koutinas, A., Cardoso, L., Pennisi, M. G., Ferrer, L., Bourdeau, P., Oliva, G., & Baneth, G. (2011). LeishVet guidelines for the practical management of canine leishmaniosis. Parasites & Vectors, 4, 86.
- Nichols, J., & Goad, D. (2020). Cutaneous vasculitis: A diagnostic and therapeutic approach. Clinician’s Brief.
- Ginel, P. J., Lucena, R., & Mozos, E. (2019). Dermal arteritis of the nasal philtrum in 23 dogs (2002-2018). Veterinary Dermatology, 30(5), 415-e125.
- Mueller, R. S., Rosenkrantz, W., Griffin, C. E., Angus, J. C., & Eichenseer, M. (2003). A retrospective study regarding the treatment of lupoid onychodystrophy in 30 dogs and literature review. Journal of the American Animal Hospital Association, 39(2), 139-150.
- Kang, M. H., Kim, J. H., & Hwang, C. Y. (2021). Successful long-term control of mucocutaneous lupus erythematosus with mycophenolate mofetil and tacrolimus in a miniature Pinscher dog. Journal of Veterinary Medical Science, 83(6), 968–972.
- Olivry, T., DeBoer, D. J., Favrot, C., Jackson, H. A., Mueller, R. S., Nuttall, T., & Prélaud, P. (2010). Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Veterinary Dermatology, 21(3), 233–248.
- Bizikova, P., & Olivry, T. (2016). A randomized, double-blinded, crossover-controlled trial of daily oral high-dose oclacitinib for the treatment of canine pemphigus foliaceus. Veterinary Dermatology, 27(5), 364-e91.
- Auxilia, S. T., & Hill, P. B. (2003). Canine symmetrical lupoid onychodystrophy: a retrospective study with particular reference to management. Journal of Small Animal Practice, 44(2), 57–62.
- Angus, J. C., & Kennis, R. A. (2006). Oral doxycycline, niacinamide and prednisolone used to treat bilateral nodular granulomatous conjunctivitis of the third eyelid in an Australian Kelpie dog. Veterinary Ophthalmology, 9(4), 259–262.
- Garcia, G., Romero, C., Sheinberg, G., et al. (2018). Three Cases of Canine Dermatomyositis-Like Disease. Acta Scientiae Veterinariae, 46(Suppl 1), 276.
Search terms
focal cutaneous vasculitis, epidermal lesions, immune mediated vasculitis, septal vasculitis, primary disease, cell poor interface dermatitis, skin disease, skin, diseases, cutaneous, vasculitis, diagnosed, cutaneous, vasculitis, treated, skin, lesions, present, cutaneous, adverse, food, reaction, ulcerative, skin, lesions, starting, life, threatening, kidney, disease, abnormal, immune, response, vasculitis, and, rare, cases, bleeding, lesions, mild, anti, inflammatory, drugs, thorough, drug, history, particularly, severe, form, blood, supply, occurs, affected, animals, veterinary, medicine, affected, animals, present