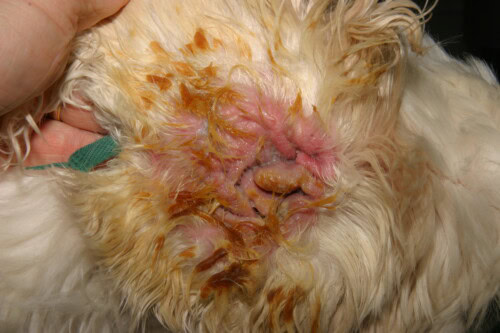
In veterinary dermatology, few microorganisms represent as constant and complex a clinical challenge as Pseudomonas aeruginosa. This Gram-negative bacillus, far from being a simple infectious agent, proves to be a formidable adversary, an opportunistic pathogen par excellence that exploits with remarkable efficiency the slightest alteration of the skin barrier or the slightest immune deficiency of the host.
Introduction
The infections it causes, primarily chronic external otitis and deep pyodermas, are often characterized by their severity, chronicity, and recalcitrant nature to treatment.
The management of these conditions is made particularly challenging by two fundamental biological characteristics of this pathogen. On one hand, P. aeruginosa possesses intrinsic resistance to numerous antibiotic classes, a capacity it complements with a remarkable ability to acquire new resistance mechanisms, leading to the emergence of multidrug-resistant (MDR) strains. On the other hand, its ability to form biofilms — structured bacterial communities protected by an extracellular matrix — constitutes a veritable fortress sheltering it from the host’s immune defenses and antimicrobial agents. This dual strategy of defense and persistence is the primary cause of therapeutic failures and frustrating recurrences for the clinician.
Facing this growing problem, a simplistic or empirical therapeutic approach is doomed to failure. The present synthesis aims to provide a comprehensive and integrated analysis, based on the most recent scientific data, for the management of dermatological infections caused by P. aeruginosa. This reference article will detail the biology and pathogenesis of this microorganism, propose a reasoned and structured diagnostic approach, and explore in depth multimodal therapeutic strategies. Particular attention will be paid to the critical interpretation of the antibiogram in light of new recommendations, the management of multidrug-resistant infections, and the evaluation of promising therapeutic innovations, such as anti-biofilm agents and phage therapy. The ambition of this work is to equip the clinician with the conceptual and practical tools necessary to transform the management of these complex cases from an uncertain battle to a controlled and effective therapeutic strategy.
1. Pseudomonas aeruginosa: Portrait of a Formidable Opportunistic Pathogen
1.1. Ecology and Microbiological Characteristics
Understanding the nature of Pseudomonas aeruginosa begins with recognizing its ubiquity. This microorganism is omnipresent in the environment, easily colonizing soil, freshwater, plant surfaces, and decomposing organic matter. This ecological plasticity explains not only its frequent presence as a simple environmental contaminant but also its leading role in nosocomial infections, where it can survive and proliferate in reservoirs as varied as disinfectants, sinks, or medical equipment. It is essential to note that P. aeruginosa is not part of the commensal microflora of the external auditory canal or healthy skin of dogs. Its presence in a dermatological sample is therefore always clinically significant.
From a microbiological standpoint, it is a Gram-negative bacillus, strict aerobe, distinguished by its motility, ensured by a single polar flagellum. Its minimal nutritional requirements and ability to tolerate a wide range of physicochemical conditions, including temperatures up to 42°C, confer exceptional survival and adaptation capacity, allowing it to colonize hostile environments where few other bacteria can subsist.
1.2. Virulence Arsenal: From Toxins to Enzymes
The pathogenic power of P. aeruginosa does not reside in a single toxin, but in a complex and redundant arsenal of virulence factors that act synergistically to invade tissues, evade the immune response, and cause cellular damage.
Among the best-known factors are its pigments. Pyocyanin, a blue-green pigment that gives its characteristic color to “pyocyanic” pus, is not simply a visual marker. It exerts powerful pro-inflammatory effects on phagocytes and impairs the immune functions of epithelial cells, actively contributing to pathogenesis. Tissue invasion is facilitated by a battery of extracellular enzymes. Elastase degrades collagen and elastin, major components of the extracellular matrix, while alkaline protease interferes with fibrin formation, preventing containment of the infection. Phospholipases degrade phospholipids in cell membranes, leading to cell lysis.
To this arsenal are added powerful toxins. The cytotoxin (or leukocidin) and hemolysins target and destroy immune cells and erythrocytes. Additionally, P. aeruginosa uses sophisticated secretion systems, such as the type III secretion system (T3SS), which acts as a molecular syringe to inject toxic effectors (exotoxins S and A) directly into the heart of host cells. These exotoxins paralyze cell functions, notably phagocytosis, and induce localized tissue necrosis, creating an environment conducive to bacterial proliferation.
1.3. Biofilm Formation: A Bacterial Fortress
Perhaps the most determining virulence factor in the chronicity of P. aeruginosa infections is its ability to form a biofilm. This is not simply a bacterial aggregation, but a highly organized microbial community embedded in a self-produced matrix of exopolysaccharides, primarily composed of alginate. This viscous matrix firmly anchors bacteria to the surface of the auditory canal or ulcerated skin, forming a veritable fortress.
This structure confers multiple advantages to the bacterium. First, it constitutes a physical barrier that protects it from phagocytic cells of the immune system and considerably limits the penetration of antibiotics. Studies have shown that bacteria organized in biofilms can be hundreds, even thousands of times more resistant to antimicrobial agents than their planktonic (free) counterparts. Second, within the biofilm, bacteria communicate with each other via a system of chemical signals called quorum sensing. This mechanism, dependent on cell density, allows them to coordinate the expression of virulence genes and defense strategies collectively, behaving like a multicellular organism.
The clinical impact of this strategy is major. The prevalence of biofilm formation by clinical isolates of P. aeruginosa from canine otitis is extremely high, reported in 40% to over 90% of cases according to studies. A large European study even revealed that 82% of isolates were capable of forming strong biofilms. This capacity is a direct cause of therapeutic failure, persistence of infection despite apparently appropriate antibiotic therapy, and the establishment of destructive chronic inflammation.
1.4. Antibiotic Resistance Mechanisms: Intrinsic and Acquired Strategies
The formidable capacity of P. aeruginosa to resist antibiotics relies on a dual strategy: natural (intrinsic) resistance and exceptional adaptability (acquired resistance).
The intrinsic resistance is an inherent characteristic of the species. It is primarily due to the low permeability of its outer membrane, which restricts the entry of numerous molecules. To this is added an arsenal of efflux pumps, membrane proteins that actively expel antibiotics out of the cell before they reach their target. Finally, P. aeruginosa constitutively expresses a chromosomal β-lactamase (AmpC), an enzyme capable of inactivating certain penicillins and cephalosporins.
The acquired resistance is even more concerning. It results either from spontaneous chromosomal mutations or, more frequently, from the acquisition of mobile genetic material (plasmids, transposons) by horizontal transfer from other bacteria. These mechanisms allow the bacterium to develop resistance to antibiotic classes to which it was initially susceptible, leading to the emergence of multidrug resistance (MDR) phenotypes. The main acquired mechanisms include the production of additional inactivating enzymes (such as extended-spectrum β-lactamases or carbapenemases), modification of the antibiotic target (for example, mutations in DNA gyrase genes conferring resistance to fluoroquinolones), or overexpression of efflux pumps.
This combination of aggressive virulence, biofilm protection, and multiple resistance mechanisms makes P. aeruginosa a particularly difficult pathogen to eradicate. Its clinical success is not the result of a single factor, but of an integrated and synergistic survival strategy. The bacterium first uses its enzymes and toxins to establish itself and damage tissues. It then builds its fortress, the biofilm, to protect itself from the first waves of host defense and treatments. Sheltered, it has time to develop and exchange resistance genes, arming itself for future assaults. Any therapeutic approach that does not account for this global strategy is inevitably doomed to failure. Treatment must therefore be multimodal, simultaneously aiming to dismantle the fortress, neutralize the pathogens, and repair the damage inflicted on the host.
Table 1: Key Virulence Factors of P. aeruginosa and Their Pathogenic Roles
|
Virulence Factor |
Category |
Main Pathogenic Role |
|
Pyocyanin |
Pigment |
Alteration of immune response, oxidative stress, pro-inflammatory effects |
|
Elastase, Alkaline protease |
Extracellular enzymes |
Destruction of extracellular matrix (collagen, elastin), tissue invasion |
|
Phospholipase C |
Extracellular enzyme |
Lysis of host cells by degradation of lipid membranes |
|
Exotoxin A, Exoenzyme S |
Toxins (T3 Secretion System) |
Inhibition of protein synthesis, apoptosis of host cells, paralysis of phagocytes |
|
Lipopolysaccharide (LPS) |
Membrane component |
Induction of strong inflammatory response, septic shock |
|
Alginate / Biofilm |
Protective matrix |
Protection against antibiotics and immune system, adhesion, chronic persistence |
|
Efflux pumps |
Resistance mechanism |
Active expulsion of multiple antibiotic classes out of the bacterial cell |
|
β-lactamases (AmpC, etc.) |
Resistance enzymes |
Enzymatic inactivation of β-lactam family antibiotics |
2. Clinical Manifestations and Epidemiology
2.1. Pseudomonas aeruginosa Otitis
Otitis caused by Pseudomonas aeruginosa represents one of the most severe and frustrating clinical entities in canine dermatology. It is crucial to understand that it is only very rarely a primary infection. It is almost systematically a complication of chronic or recurrent external otitis, initially caused by proliferation of other agents, typically yeasts (Malassezia pachydermatis) or cocci (Staphylococcus pseudintermedius). Progression to Pseudomonas infection is a true pathological escalation, favored by a set of factors. Predisposing factors (ear conformation with a narrow canal or pendulous pinna, excessive humidity), primary factors (the initial cause of inflammation, such as atopic dermatitis, food allergy, or foreign body), and perpetuating factors (pathological changes of the canal such as ceruminous gland hyperplasia, stenosis, or rupture of the tympanic membrane) create an ideal microenvironment for colonization by this opportunistic pathogen.
Severe Pseudomonas Otitis in a Cocker Spaniel
The clinical presentation is often characteristic. The condition is frequently unilateral, although it can be bilateral in the context of underlying allergic disease. It manifests with intense aural pain, often more pronounced than pruritus, severe erythematous inflammation of the canal, and ulcerations of the epithelium. The most evocative sign is the nature of the exudate: it is typically abundant, mucoid or purulent, malodorous, and of a characteristic yellow-greenish color due to the production of bacterial pigments. In severe cases, this exudate may be hemorrhagic. Careful examination of the pinna and ear canal entrance may reveal the presence of sticky patches, a direct clinical manifestation of bacterial biofilm. A major and frequent complication is extension of the infection to the middle ear. It is estimated that over 80% of dogs with chronic external otitis caused by Pseudomonas have concurrent middle ear otitis. This can manifest with neurological signs such as Horner’s syndrome, facial nerve paralysis, or vestibular disorders.
From an epidemiological standpoint, external otitis is an extremely common condition, affecting between 10% and 20% of the canine population. Within this population, P. aeruginosa stands out as the most frequently isolated pathogen in chronic and recalcitrant cases, being associated with nearly 35% of these severe forms. Other studies, conducted on specific populations of dogs presenting with otitis, report prevalences ranging from 25% to over 50%.
2.2. Pseudomonas aeruginosa Pyoderma
Although less frequent than otitis, pyodermas caused by P. aeruginosa are often spectacular and serious conditions. They generally present in two main forms.
The acute deep pyoderma is the most dramatic form. It is characterized by the sudden appearance of extremely painful lesions, classically located along the dorsal line. Clinical examination reveals multiple circinate ulcers (ring-shaped), hemorrhagic crusts, and furuncles that may ooze a serosanguinous exudate. The general condition of the animal is often affected, with lethargy and fever.
The intertrigo (or skin fold dermatitis) caused by Pseudomonas is another presentation. It develops in areas of skin folds where maceration and friction create favorable conditions (facial folds in brachycephalic breeds, tail fold, vulvar folds). Lesions consist of erosions and deep ulcerations, covered with a greenish, thick, and particularly sticky exudate, highly suggestive of this organism’s involvement.
The exact prevalence of Pseudomonas pyodermas is less well documented than that of otitis. However, studies on canine skin infections have identified P. aeruginosa in approximately 11% to 13% of cases. A study conducted in Romania on superficial skin infections isolated the organism in nearly 34% of pyoderma samples.
The occurrence of P. aeruginosa infection, whether in the ear or on the skin, should be interpreted by the clinician as a warning signal. It is not a fortuitous event, but the culmination of a pathological cascade. Chronicity is the main risk factor. Persistent inflammation, humidity, and especially repeated antibiotic treatments, often broad-spectrum, create intense selection pressure. They eliminate the protective commensal flora and more sensitive pathogens, leaving the field open to P. aeruginosa, intrinsically more resistant and perfectly adapted to this hostile and modified environment. Consequently, identification of P. aeruginosa is not the end of diagnosis, but the beginning of a more thorough investigation. It requires rigorous investigation of the primary cause (allergy, endocrine disorder, etc.) that allowed this “crisis” to establish itself. Without management of this initial factor, any attempt to treat the Pseudomonas infection, even with the most powerful antibiotic, will only be temporary respite before inevitable recurrence.
3. Reasoned Diagnostic Approach
Effective management of P. aeruginosa infection relies on a structured and hierarchical diagnostic approach. The objective is not only to identify the pathogen but also to understand the context of its development, assess the extent of lesions, and guide targeted and rational therapy.
3.1. Clinical and Otoscopic Examination: Crucial Initial Steps
Any diagnostic approach begins with rigorous history-taking and complete physical examination. Questioning of the owner should seek signs of underlying diseases, particularly pruritus outside the ears, digestive disorders, or recurrent skin infections that may suggest atopic dermatitis or food allergy. Signs of polyuria-polydipsia or weight gain may point to an endocrinopathy. The dermatological examination must be comprehensive, as otitis is often only one manifestation of a more global dermatosis.
The otoscopic examination is a fundamental step. Use of a video-otoscope is ideal, as it offers superior magnification, lighting, and recording capability, facilitating precise evaluation and follow-up. Otoscopy allows assessment of the external auditory canal condition: degree of erythema, presence of ulcerations, tissue proliferations (stenosis), or masses. It is also indispensable for evaluating the integrity of the tympanic membrane. A ruptured, bulging, or discolored membrane is a strong sign of middle ear involvement. Visualization of purulent exudate and ulcers is highly suggestive of Gram-negative bacilli infection.
3.2. Cytological Examination: A First-Line Diagnostic Tool
The cytological examination of aural material is the most important, fastest, and most cost-effective complementary examination in otitis management. It should be performed systematically at each consultation for otitis. The sample is taken using a swab, attempting to reach the junction between the vertical and horizontal canals, where secretions are most representative of the infection. The sample is then smeared on a slide, stained (for example, with rapid Romanowsky-type staining), and examined under microscope.
In the context of P. aeruginosa infection, results are often characteristic. Observation of rod-shaped bacteria (bacilli), often in large numbers, is the main alert sign. These bacilli are typically accompanied by a dense inflammatory population, mainly composed of degenerate neutrophils, indicating active and suppurative infection. Cytology provides almost immediate diagnostic and therapeutic guidance, allowing initiation of targeted topical treatment against Gram-negative bacilli without waiting for culture results.
3.3. Bacterial Culture and Antibiogram: Indications and Sampling Protocol
The bacterial culture with antibiogram is not a routine examination for a first uncomplicated otitis. Its prescription should be reasoned and reserved for specific clinical situations where it provides crucial information for subsequent treatment:
- Confirmed presence of bacilli on cytological examination, particularly if the infection is chronic or recurrent.
- Failure of a well-conducted first-line empirical treatment.
- Need to consider systemic antibiotic therapy, particularly in cases of confirmed middle ear otitis or associated deep pyoderma. In this case, the antibiogram is essential to guide molecule selection.
The sampling protocol is critical for result reliability. The sample must be collected as aseptically as possible, ideally from the deep portion of the auditory canal. When middle ear otitis is suspected with an intact tympanic membrane, the only way to obtain a representative sample from the tympanic bulla is to perform a myringotomy. This procedure, performed under general anesthesia and video-otoscopic guidance, consists of sterile perforation of the tympanum to aspirate the middle ear content for cytological analysis and culture.
3.4. Medical Imaging in Middle Ear Otitis Exploration
When middle ear involvement is suspected (based on clinical signs, otoscopy, or therapeutic failure), medical imaging becomes an essential diagnostic tool. It allows confirmation of involvement, assessment of severity, and planning of possible surgical intervention.
The CT scan (computed tomography or CT-scan) is considered the imaging modality of choice. It offers excellent resolution for visualizing bony structures, notably the tympanic bulla wall (searching for bone lysis) and ossicles, as well as for detecting the presence of fluid or abnormal soft tissue in the middle ear. Magnetic resonance imaging (MRI) is an alternative that offers better soft tissue visualization and is particularly indicated in cases of suspected tumor mass or infection extension to surrounding nerve structures.
This sequential approach, ranging from rapid cytology to advanced imaging, embodies a rational diagnostic approach. Each complementary examination is not prescribed randomly but aims to answer a specific clinical question that arises at a given stage of the process. Cytology guides initial treatment. Failure of this treatment raises the question of resistance (answered by culture) or anatomical extension (answered by CT scan). This hierarchy prevents unjustified therapeutic escalation and promotes reasoned use of antibiotics and expensive examinations.
4. Integrated Therapeutic Strategies
Treatment of P. aeruginosa infections is a demanding process requiring a multimodal and persevering approach. Success does not rely on a single “miracle” molecule but on an integrated strategy aimed at simultaneously eliminating the pathogen, controlling inflammation, correcting underlying factors, and restoring a healthy aural environment.
4.1. Fundamental Principles: Primary Cause Management and Inflammation Control
The most fundamental principle, yet sometimes neglected, is that long-term treatment of Pseudomonas infection is inseparable from identification and management of the primary cause of otitis. Whether it is an allergy, endocrinopathy, or mass, its control is the only guarantee against recurrences. Pseudomonas infection is a consequence; treating only the consequence without addressing the cause is a therapeutic dead end.
In parallel, controlling inflammation is a pillar of treatment. Chronic inflammation causes edema, pain, sebaceous and ceruminous gland hyperplasia, and canal stenosis. These changes perpetuate infection and prevent penetration of topical treatments. Use of glucocorticoids, topically and/or systemically (for example, prednisolone at anti-inflammatory dose), is therefore crucial in the initial phase. They allow “reopening” of the canal, decrease exudate production, improve the animal’s comfort, and consequently increase antimicrobial agent efficacy.
4.2. Topical Therapy: Cornerstone of Treatment
Topical therapy is at the heart of the therapeutic strategy, as it alone allows achieving antimicrobial concentrations at the infection site that are far superior to those obtained systemically. Its efficacy depends on two key steps: cleaning and application of targeted agents.
The aural cleaning is a non-negotiable step. Purulent exudate and biofilm act as a physical barrier and can chemically inactivate certain antibiotics, notably aminoglycosides. Deep initial cleaning, performed under general anesthesia with abundant irrigation, is often essential to remove the majority of debris. Subsequently, regular home cleanings by the owner are necessary to keep the canal clean.
The efficacy of topical therapy can be considerably increased by using solutions containing Tris-EDTA. EDTA (ethylenediaminetetraacetic acid) is a chelating agent that binds to metallic ions (calcium, magnesium) essential for the stability of the outer membrane of Gram-negative bacteria. By destabilizing this membrane, Tris-EDTA makes it more permeable to antibiotics, thus potentiating their action. Studies have shown that it significantly increases the efficacy of fluoroquinolones and aminoglycosides against P. aeruginosa. The optimal protocol consists of instilling the Tris-EDTA solution into the auditory canal and waiting 20 to 30 minutes before applying the antibiotic medication, to allow time for the chelating agent to act.
The choice of topical antimicrobial agent must be thoughtful. Several options are available:
- The fluoroquinolones (marbofloxacin, enrofloxacin) and aminoglycosides (gentamicin, amikacin) are common and often effective choices. Caution is warranted with aminoglycosides in case of tympanic rupture due to their potential ototoxicity.
- The polymyxin B is another effective antibiotic against P. aeruginosa.
- The silver sulfadiazine (SSD) is a particularly interesting option, especially against multidrug-resistant strains. As a 1% cream, it has demonstrated excellent in vitro activity against canine isolates of P. aeruginosa, with very low minimal inhibitory concentrations (MICs) (1-64 µg/mL), well below the concentration of commercial preparation. Its mechanism of action, targeting the cell wall and membrane, is different from that of many other antibiotics.
4.3. Systemic Antibiotic Therapy: Indications, Choices, and Risks
Systemic antibiotic therapy is not a first-line treatment for external otitis, even Pseudomonas-associated. Concentrations achieved in cerumen and canal epithelium are often insufficient. Its use should be reserved for specific and justified indications:
- Middle ear otitis confirmed by imaging or otoscopy.
- Associated deep pyoderma.
- Inflammation and tissue hyperplasia so severe that they prevent any effective topical application.
- Proven failure of aggressive and well-conducted topical treatment.
The choice of molecule must imperatively be guided by an antibiogram.
- The fluoroquinolones are the only class available orally with reliable activity. However, high doses, off-label, are required to be effective against P. aeruginosa: enrofloxacin at 10-20 mg/kg/day and marbofloxacin at 5.5 mg/kg/day. It should be noted that resistance can develop rapidly during treatment.
- Parenteral (injectable) options include third-generation cephalosporins (such as ceftazidime) and aminoglycosides (amikacin, gentamicin). Systemic use of aminoglycosides is limited by their risk of nephrotoxicity and ototoxicity, and requires perfect animal hydration as well as renal function monitoring.
- The carbapenems (imipenem, meropenem) and anti-pseudomonal penicillins (ticarcillin) are last-resort antibiotics, to be used only for documented multidrug-resistant infections, in compliance with antibiotic stewardship principles to preserve their efficacy in human and veterinary medicine.
- Note that these latter molecules are to be used according to regulations in force and recommendations of each country, GEDAC for France for example.
Table 2: Dosages of Systemic Anti-Pseudomonal Antibiotics in Dogs
|
Molecule |
Class |
Dosage (route) |
Clinical Comments / Precautions |
|
Enrofloxacin |
Fluoroquinolone |
10–20 mg/kg/day (PO, IV, SC) |
Requires high doses. Resistance can develop rapidly. |
|
Marbofloxacin |
Fluoroquinolone |
5.5 mg/kg/day (PO) |
Maximum dose required for efficacy against strains with intermediate susceptibility. |
|
Amikacin |
Aminoglycoside |
15–30 mg/kg/day (IV, IM, SC) |
Risk of nephrotoxicity and ototoxicity. Ensure good hydration and monitor renal function. |
|
Gentamicin |
Aminoglycoside |
10–14 mg/kg/day (IV, IM, SC) |
Higher toxicity risk than amikacin. Monitoring required. |
4.4. Critical Interpretation of the Antibiogram According to CLSI VET Standards
The antibiogram is a powerful tool, but its interpretation should not be passive. The Clinical and Laboratory Standards Institute (CLSI) has recently published a major update of critical thresholds (breakpoints) for fluoroquinolones in dogs, which radically changes their interpretation.
The old, higher thresholds could classify as “Susceptible” (S) a strain that would not respond to standard-dose treatment. The new thresholds are much lower and introduce a new category: “Susceptible-Dose Dependent” (SDD). This category indicates that a strain can be successfully treated, but only if the highest dose of the approved dosage range is used.
This evolution transforms the antibiogram from a simple “S/I/R” report into a true prescribing guide. Facing an SDD-classified strain, the clinician knows they must prescribe the maximum recommended dose (for example, 20 mg/kg/day for enrofloxacin) to have a chance of clinical success. Using a standard dose against an SDD strain is a predictable cause of therapeutic failure and resistance selection.
Table 3: Revised CLSI VET Critical Thresholds (2023) for Enrofloxacin and Marbofloxacin in Dogs (Oral Route)
|
Antibiotic |
Category and MIC Threshold (µg/mL) |
Corresponding dose |
|
Enrofloxacin (revised) |
S ≤ 0.06 |
5 mg/kg/day |
|
SDD 0.12–0.25 |
10–20 mg/kg/day |
|
|
R ≥ 0.5 |
– |
|
|
Marbofloxacin (revised) |
S ≤ 0.12 |
2.8 mg/kg/day |
|
SDD 0.25 |
5.5 mg/kg/day |
|
|
R ≥ 0.5 |
– |
Ultimately, therapeutic strategy should not be viewed as a simple “antibiotic battle” but as “ecosystem management.” The objective is not only to eradicate P. aeruginosa, but to restore a cutaneous or aural environment in which it can no longer proliferate. Cleaning, Tris-EDTA, and anti-inflammatories modify the physical, chemical, and immunological environment. Treatment of the primary cause corrects the fundamental imbalance. In this scheme, antibiotics are only a tool, often temporary, to reduce bacterial load while other measures restore balance. Success is measured less by cure of the infectious episode than by prevention of its recurrence.
5. Management of Multidrug-Resistant Infections and Future Perspectives
The emergence of multidrug-resistant (MDR) strains of P. aeruginosa constitutes the ultimate challenge in veterinary dermatology. Faced with an antibiogram showing few or no susceptibilities, the clinician must abandon the idea of a simple solution and adopt alternative and combined strategies, while turning to therapeutic innovations that are redefining the fight against bacterial infections.
5.1. Combined Strategies for Multidrug-Resistant (MDR) Strains
Management of an MDR P. aeruginosa infection relies on intensification of the multimodal approach. Each treatment component becomes critical. Rigorous and repeated mechanical cleaning, often under anesthesia, is the first step to physically dismantle the biofilm and reduce bacterial load. Systematic use of Tris-EDTA as pretreatment becomes not an option but a necessity to attempt to restore susceptibility, even partial, to antibiotics.
The choice of topical agents must be creative and based on the few remaining options. Silver sulfadiazine is often an excellent empirical choice in this context. Extemporaneous preparation of topical solutions based on injectable antibiotics, such as ticarcillin or amikacin, can be considered, although the stability of these preparations is a concern. Systemic antibiotic therapy, if absolutely necessary, must be scrupulously guided by the antibiogram, considering last-resort molecules after rigorous benefit/risk assessment.
5.2. Biofilm Disruption: The Role of N-acetylcysteine (NAC)
Recognition of biofilm as a fortress protecting bacteria has paved the way for research into “anti-biofilm” agents. N-acetylcysteine (NAC), a well-known mucolytic agent, has emerged as a very promising candidate. In vitro studies have demonstrated that NAC possesses not only direct antibacterial activity against otitis pathogens, including P. aeruginosa, but is especially capable of disrupting the biofilm matrix, degrading it and thus exposing the bacteria it shelters.
The in vitro data are convincing: NAC inhibits P. aeruginosa growth at minimal inhibitory concentrations (MICs) of 5 to 20 mg/mL, levels considered safe and achievable with topical application. Its combination with Tris-EDTA could offer a synergy of action, Tris-EDTA weakening the bacterial wall while NAC dismantles the extracellular matrix. Although prospective clinical trials are still needed to validate its in vivo efficacy, NAC represents an adjuvant therapeutic option of great interest for chronic and biofilm infections, in addition to antimicrobial treatments.
5.3. Innovative Therapies: Phage Therapy as a Promising Alternative
Faced with the impasse of antibiotic resistance, the scientific community is turning to radically different approaches, and phage therapy is one of the most promising. This technique uses bacteriophages (or phages), viruses that are the natural predators of bacteria. So-called “lytic” phages infect a specific target bacterium, replicate inside, then burst it (lysis), releasing dozens of new viral particles ready to infect neighboring bacteria.
The advantages of this approach are multiple. Phages possess high specificity, targeting only the pathogenic bacterium without affecting the host’s beneficial microflora. They have the ability to self-replicate at the infection site as long as their target is present. Above all, they are effective against multidrug-resistant bacteria, as their mechanism of action is completely independent of antibiotic susceptibility. Moreover, many phages produce enzymes capable of degrading biofilm matrix, allowing them to penetrate this protective structure.
The phage therapy is currently an extremely active research area in veterinary medicine. In vitro studies and published case reports have shown very encouraging results in treating skin infections, otitis, and surgical wound infections caused by MDR P. aeruginosa in dogs and cats. Clinical trials are underway to rigorously evaluate the efficacy and safety of phage cocktails. The synergistic combination of phages and antibiotics is also being explored, where the phage weakens the bacterial population, making it more vulnerable to the antibiotic.
The fight against MDR P. aeruginosa illustrates a major strategic evolution. We are moving from a “brute force” logic, consisting of seeking ever more powerful antibiotics, to an “asymmetric warfare” logic, which aims to exploit the structural and biological weaknesses of the bacterium. Instead of trying to break through the fortress walls with a bigger battering ram, new strategies aim to dissolve the cement holding the stones (NAC) or send specialized agents that can bypass defenses (phages). The future of managing these infections probably does not lie in a single “super-antibiotic,” but in the art of intelligently combining these new weapons to dismantle the sophisticated defenses of this formidable adversary.
Conclusion and Future Research Directions
Management of pyodermas and otitis caused by Pseudomonas aeruginosa in dogs represents a paradigm of modern infectious medicine, where understanding the pathogen’s biology and its interactions with the host is the key to therapeutic success. This synthesis has highlighted that P. aeruginosa is not a simple infectious agent, but a highly adaptive opportunistic pathogen, whose effective management transcends simple antibiotic prescription. Success relies on a holistic and meticulous approach, whose pillars are: accurate and hierarchical diagnosis, initiated by cytology; imperative control of the underlying primary cause and inflammation; aggressive topical therapy combining mechanical cleaning and potentiating agents such as Tris-EDTA; and judicious and reasoned use of systemic antibiotic therapy, guided by an antibiogram interpreted in light of the most recent standards and pharmacodynamic concepts.
The era of empirical and prolonged antibiotic therapy is ending, replaced by a strategic approach that integrates cutaneous and aural ecosystem management. However, many questions remain and significant challenges, notably the rise of multidrug resistance, require continuous and innovative research. Several future research directions appear as priorities to improve clinical management:
- Development and clinical validation of synergistic topical formulations: It is crucial to conduct prospective, randomized, and controlled clinical trials to evaluate the in vivo efficacy of formulations combining anti-biofilm agents (such as N-acetylcysteine) and antimicrobials. Demonstration of clinical synergy would establish new treatment standards for chronic infections.
- Clinical trials on phage therapy: Phage therapy represents a promising alternative, but its potential must be validated by rigorous studies. Establishment of well-characterized phage banks and conduct of clinical trials evaluating the efficacy and safety of phage cocktails for treating MDR P. aeruginosa infections are an absolute priority.
- Study of the aural microbiome: Better understanding of the dysbiosis that precedes and favors colonization by P. aeruginosa is necessary. Analysis of the aural microbiome in healthy and atopic dogs could identify risk markers and explore the potential of microbiome-modulating therapies, such as topical probiotics, in prevention.
- Development of rapid diagnostic tools: Development of rapid point-of-care diagnostic tests, capable of detecting within minutes not only the presence of P. aeruginosa but also key genetic resistance markers, would allow institution of targeted therapy much earlier, improving prognosis and limiting selection pressure.
- Pharmacokinetics and pharmacodynamics of topical treatments: Data on diffusion and persistence of topical antimicrobial agents in the inflamed auditory canal and in the presence of biofilm are surprisingly scarce. Targeted PK/PD studies are needed to optimize concentrations, formulations, and administration frequencies to maximize efficacy and minimize resistance emergence.
In conclusion, while Pseudomonas aeruginosa remains a formidable adversary, better understanding of its biology, combined with a rigorous clinical approach and integration of innovative therapies, offers encouraging prospects for significantly improving the prognosis of these complex conditions.
References
Buckley, L. M., McEwan, N. A., & Nuttall, T. (2013). Tris-EDTA significantly enhances antibiotic efficacy against multidrug-resistant Pseudomonas aeruginosa in vitro. Veterinary Dermatology, 24(5), 519-e122.
Carlotti, D. N., Ghibaudo, G., & Damborg, P. (2010). In vitro antimicrobial activity of a commercial ear antiseptic containing chlorhexidine and Tris-EDTA. Veterinary Dermatology, 21(3), 282-286.
Cole, L. K., Kwochka, K. W., Kowalski, J. J., & Hillier, A. (2008). Comparison of enrofloxacin and marbofloxacin for the treatment of canine Pseudomonas otitis. Veterinary Therapeutics, 9(2), 141-151.
Fusconi, G., Ghibaudo, G., & Damborg, P. (2011). In vitro activity of a commercial ear antiseptic on planktonic and biofilm-embedded Pseudomonas aeruginosa. Veterinary Dermatology, 22(4), 366-372.
Hattab, J., et al. (2021). Prevalence of five virulence genes in Pseudomonas aeruginosa isolates from canine infections. Journal of Applied Microbiology, 130(5), 1548-1556.
Hawkins, C., Harper, D., & Soothill, J. (2010). Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: a before/after clinical trial. Veterinary Microbiology, 146(3-4), 309-313.
Hillier, A., et al. (2023). Revision of the CLSI antimicrobial susceptibility testing breakpoints for enrofloxacin and marbofloxacin in dogs. American Journal of Veterinary Research, 84(11).
May, E. R., Conklin, K. A., & Bemis, D. A. (2016). Antibacterial effect of N-acetylcysteine on common canine otitis externa isolates. Veterinary Dermatology, 27(3), 188-e47.
Morris, D. O., et al. (2023). A retrospective study of the primary causes of Pseudomonas otitis in 60 dogs. Journal of Small Animal Practice, 64(4), 241-247.
Nuttall, T., & Cole, L. K. (2007). Evidence-based veterinary dermatology: a systematic review of interventions for treatment of Pseudomonas otitis in dogs. Veterinary Dermatology, 18(2), 69-77.
Pye, C. (2018). Surgical management of end-stage otitis in dogs. Veterinary Clinics: Small Animal Practice, 48(1), 75-91.
Robinson, A., et al. (2019). Genomic and phenotypic characterisation of Pseudomonas aeruginosa isolates from canine otitis externa. Frontiers in Microbiology, 10, 1234.
Secker, B., et al. (2023). A multinational European survey of Pseudomonas aeruginosa isolates from canine otitis: antimicrobial resistance, biofilm formation, and virulence gene profiles. Frontiers in Microbiology, 14, 1526843.
Wright, A., Hawkins, C., Änggård, E. E., & Harper, D. R. (2009). A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clinical Otolaryngology, 34(4), 349-357.
Yoon, J., & Park, C. (2024). In vitro synergistic efficacy of N-acetylcysteine with antimicrobial agents against Pseudomonas aeruginosa isolated from dogs with otitis externa. Korean Journal of Veterinary Research, 64(1), e5.