A review centered on German Shepherd Dog skin problems synthesizes information on genetic predisposition, immune dysregulation, diagnostic strategies (cytology, histopathology, allergy testing), and management protocols for conditions like pyoderma and atopy, offering a comprehensive resource for vets
July 2025
Introduction to Dog Skin Issues
Dermatological disorders represent a substantial proportion of the caseload in small animal veterinary practice, with various studies indicating that skin-related complaints account for a significant percentage of consultations, ranging from approximately 8% to as high as 30% of all canine cases presented. Among the myriad of clinical signs, pruritus stands as the most frequent and compelling reason for owners to seek veterinary intervention. The impact of these conditions on patient quality of life, and the diagnostic and therapeutic challenges they present, underscore their clinical importance.
Within this context, the German Shepherd Dog (GSD) emerges not merely as another affected breed but as a sentinel for studying the intricate interplay between genetic predisposition, immunological function, and environmental influence in the development of skin disease. Epidemiological data consistently identify the GSD as a breed with a statistically significant overrepresentation for a spectrum of dermatological conditions. This predisposition is not a random collection of unrelated ailments; rather, it suggests a more fundamental, heritable dysregulation of cutaneous homeostasis. The thesis of this review is that the dermatological vulnerability of the German Shepherd Dog is rooted in a triad of factors: a genetically determined compromise in epidermal barrier function, inherent deficiencies in cutaneous immune defense, and a profound, systemic predisposition to allergic inflammation. These elements frequently converge, creating a cascade of pathology that manifests as chronic, recurrent, and often severe skin disease.
This article will provide a comprehensive and systematic analysis of the dermatological challenges specific to the German Shepherd Dog. It will deconstruct the etiologies of the most prevalent conditions, from common allergic and infectious dermatoses to specific autoimmune diseases. A detailed exploration of modern diagnostic pathways—spanning foundational in-clinic procedures like cytology to advanced histopathology and allergy testing—will be presented. Finally, current and emerging therapeutic strategies will be reviewed, encompassing pharmacological protocols, targeted immunomodulation, and dietary management. The overarching goal is to equip the veterinary professional with a deep, mechanistic understanding of GSD skin disease, emphasizing the necessity of a multimodal approach and the critical role of early, accurate diagnosis in mitigating the chronicity and morbidity associated with these complex conditions.
Common Skin Conditions
The German Shepherd Dog is susceptible to the full range of dermatoses affecting the canine species, including those of parasitic, allergic, infectious, and neoplastic origin. However, the breed exhibits a distinct pattern of prevalence and clinical severity, particularly concerning bacterial skin infections and the sequelae of allergic disease. A defining characteristic of GSD dermatology is the tendency for conditions to be recurrent, refractory to conventional short-course therapies, and underpinned by complex immunological disturbances.
A paramount condition is German Shepherd Dog Pyoderma (GSP), a term that describes a breed-specific presentation of deep bacterial folliculitis and furunculosis. GSP is not a primary disease but rather the clinical endpoint of one or more underlying triggers. It is characterized by its chronicity, severity, and a predilection for middle-aged dogs. The clinical picture is often dramatic, with intense pruritus, pain, and deep, draining lesions. The distribution classically begins over the lumbosacral dorsum, lateral stifles, and ventral abdomen before potentially progressing to a generalized state. Compared to deep pyoderma in other breeds, GSP is frequently more aggressive and painful, causing significant morbidity.
The pathophysiology of GSP is multifactorial, highlighting the syndromic nature of skin disease in this breed. It is invariably secondary to a primary trigger that disrupts cutaneous integrity and immune surveillance. Documented underlying causes include canine atopic dermatitis (CAD), cutaneous adverse food reactions (CAFR), flea allergy dermatitis (FAD), and endocrinopathies such as hypothyroidism. Beyond these triggers, GSP is associated with a unique immunological profile. Affected dogs often demonstrate significant deficiencies in mucosal immunity, evidenced by low serum Immunoglobulin A (IgA) concentrations. Concurrently, they may exhibit elevated serum Immunoglobulin G (IgG) and, critically, a disturbed cell-mediated immune response characterized by a decreased population of CD4+ helper T-lymphocytes and an abnormally elevated population of CD8+ cytotoxic/suppressor T-lymphocytes. This inverted CD4/CD8 ratio suggests a state of functional immunosuppression that impairs the ability to control bacterial proliferation. The strong breed signal implies a heritable basis for this constellation of immune defects.
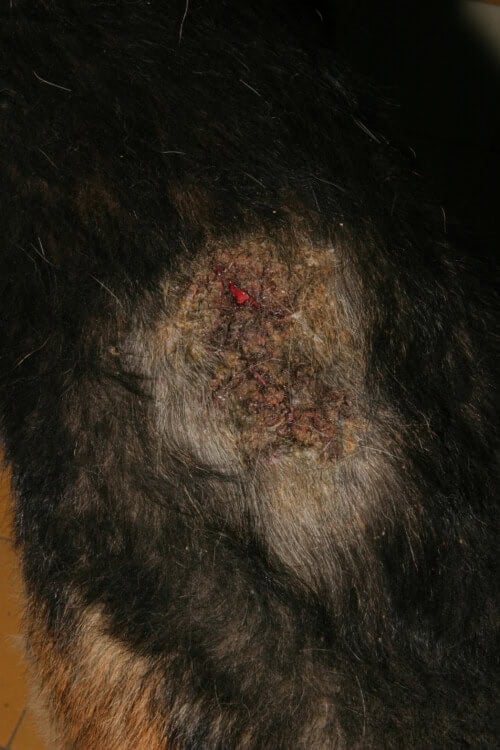
Severe dorsolumbar pyoderma due to flea allergy dermatitis
Microbiologically, the principal pathogen isolated from GSP lesions is Staphylococcus pseudintermedius. The chronicity and recurrent nature of the disease, often necessitating repeated courses of antibiotics, has driven the emergence of antimicrobial resistance. Methicillin-resistant S. pseudintermedius (MRSP) is an increasingly prevalent and serious complication, rendering empirical antibiotic selection unreliable and often ineffective. Management of GSP therefore mandates an aggressive, multi-pronged approach. This includes long-term systemic antibiotic therapy, with drug selection guided by bacterial culture and antimicrobial susceptibility testing. Adjunctive topical therapy with potent antiseptic agents, such as shampoos containing 2-4% chlorhexidine, is essential to reduce surface bacterial load and support barrier health. Most importantly, successful long-term control depends on the definitive diagnosis and rigorous management of the underlying primary allergic or endocrine disease.
Table 1: Key Dermatological Conditions in the German Shepherd Dog
|
Condition |
Primary Pathophysiology |
Key Clinical Signs in GSDs |
Diagnostic Hallmarks |
|
German Shepherd Pyoderma (GSP) |
Immune dysregulation (low IgA, T-cell imbalance) secondary to an allergic or endocrine trigger. |
Deep, painful, pruritic furunculosis and draining tracts; often begins on the lumbosacral region, thighs, and ventrum. |
Cytology: pyogranulomatous inflammation with intracellular cocci. Histopathology: suppurative to pyogranulomatous furunculosis. Bacterial culture is mandatory. |
|
Canine Atopic Dermatitis (CAD) |
Genetic skin barrier defect (e.g., PKP2 mutation) combined with a Th2-skewed immune response to environmental allergens. |
Intense pruritus, erythema, alopecia, excoriations, and secondary lichenification. Characteristic distribution on elbows, thorax, paws, and groin. |
Clinical diagnosis based on history and exclusion of other pruritic diseases (e.g., Favrot’s criteria). Intradermal or serum allergy testing is used to guide immunotherapy, not for diagnosis. |
|
Acute Moist Dermatitis (“Hot Spot”) |
Focal, self-trauma-induced pyotraumatic dermatitis, often triggered by an underlying pruritic stimulus like a flea bite or allergy. |
A rapidly appearing, well-demarcated, erythematous, exudative, alopecic, and painful lesion. |
Distinct clinical appearance. Cytology reveals suppurative inflammation with high numbers of bacteria (cocci). |
|
Malassezia Dermatitis |
Overgrowth of the commensal yeast Malassezia pachydermatis, typically secondary to underlying allergic, endocrine, or keratinization disorders. |
Erythema, greasy or waxy exudate, malodor, hyperpigmentation, and lichenification. Commonly affects skin folds, ventrum, paws, and claw beds. |
Cytology is definitive, revealing characteristic “peanut” or “footprint” shaped yeast organisms. |
|
Generalized Demodicosis |
Proliferation of Demodex canis mites, which are normal fauna, due to an underlying compromise of the host’s immune system. |
Multifocal to diffuse alopecia, erythema, scaling, and comedones (“blackheads”). Secondary bacterial pyoderma is a frequent and severe complication. |
Deep skin scrapings or trichogram revealing high numbers of mites in all life stages (adults, larvae, eggs). |
Effective management of bacterial pyoderma, a cornerstone of GSD dermatology, relies on judicious antimicrobial selection. The rise of MRSP necessitates a structured approach to therapy, prioritizing narrower-spectrum agents for initial treatment of uncomplicated infections and reserving broader-spectrum drugs for cases guided by culture results.
Table 2: First- and Second-Line Systemic Antimicrobials for Canine Pyoderma
|
Antimicrobial Class |
Drug Name |
Key Considerations & Cautions |
|
First-Line (Tier 1) |
For empirical treatment of suspected methicillin-susceptible S. pseudintermedius (MSSP) superficial pyoderma. |
|
|
Cephalosporins |
Cephalexin |
Excellent safety profile. Good first choice for superficial pyoderma. |
|
Lincosamides |
Clindamycin |
Good penetration. Crucially, do not use if culture shows resistance to erythromycin, as this may indicate inducible clindamycin resistance (a D-test can confirm). |
|
Potentiated Penicillins |
Amoxicillin-Clavulanate |
Broad spectrum; effective against Staphylococci. Potential for GI upset. |
|
Second-Line (Tier 2) |
Use should be strictly guided by culture and antimicrobial susceptibility testing, especially for deep or recurrent pyoderma. |
|
|
Potentiated Sulfonamides |
Trimethoprim-Sulfamethoxazole |
Can cause keratoconjunctivitis sicca (KCS), hypothyroidism, and arthropathy (especially in Dobermans). Monitor tear production. |
|
Fluoroquinolones |
Marbofloxacin, Enrofloxacin, Pradofloxacin |
Reserve for documented multidrug-resistant infections to preserve efficacy. Ciprofloxacin has poor/erratic bioavailability in dogs and is not recommended. |
|
Tetracyclines |
Doxycycline |
Use only if susceptibility is confirmed. Resistance is common. Can cause GI upset; administer with food. |
Allergic Reactions
The German Shepherd Dog stands as an archetypal breed for allergic skin disease, exhibiting an exceptionally high genetic predisposition to developing canine atopic dermatitis (CAD). This chronic, inflammatory, and intensely pruritic condition typically manifests between the ages of six months and three years, setting the stage for a lifetime of clinical management. The breed’s susceptibility is not merely a matter of chance but is rooted in profound, heritable defects in both the structure of the skin and the function of the immune system.
The modern understanding of CAD pathophysiology in the GSD and other predisposed breeds is best described by a “two-hit” or “outside-in, inside-out” hypothesis, which integrates defects in both the epidermal barrier and the immune response. The primary defect, or “first hit,” is a compromised skin barrier. Groundbreaking genetic research in GSDs has identified a significant association between CAD and a locus on canine chromosome 27, which harbors the Plakophilin 2 (PKP2) gene. Plakophilin 2 is a critical component of desmosomes, the intercellular junctions that provide structural integrity to the epidermis. A dysfunctional PKP2 gene leads to weakened desmosomes, resulting in a porous or “leaky” epidermal barrier. This structural failure has two major consequences: increased transepidermal water loss (TEWL), leading to xerosis (dry skin), and, more critically, enhanced percutaneous penetration of environmental allergens and microbes.
This barrier breach allows allergens—such as house dust mites, storage mites, pollens, and mold spores—to bypass the skin’s physical defenses and encounter underlying immune cells. This triggers the “second hit”: a dysregulated and exaggerated immune response. In atopic individuals, this response is strongly polarized towards a T-helper 2 (Th2) phenotype. This leads to the overproduction of a specific suite of pro-inflammatory and pruritic cytokines, including Interleukin-4 (IL-4), Interleukin-13 (IL-13), and, pivotally for the sensation of itch, Interleukin-31 (IL-31). These cytokines drive the recruitment of inflammatory cells, promote the production of allergen-specific Immunoglobulin E (IgE) by B-cells, and directly stimulate sensory nerves to cause pruritus. The GSD’s inherent tendency towards IgA deficiency may further compound this issue by reducing the capacity of the mucosal immune system to neutralize antigens at the skin surface, thereby increasing the inflammatory load. This combination of a physically weak barrier and a hyper-reactive immune system creates a self-perpetuating cycle of itching, scratching, further barrier damage, and escalating inflammation.
The clinical manifestation of CAD in the GSD is distinctive. The hallmark is an intense, often relentless pruritus that precedes the development of visible lesions. Owners typically report behaviors such as face rubbing, foot licking and chewing, and scratching of the axillae and groin. The characteristic distribution pattern of lesions in this breed involves the elbows, ventral thorax, ventral abdomen, paws (interdigital spaces), and groin. Primary lesions are often limited to erythema, but the ensuing self-trauma quickly leads to a cascade of secondary lesions, including excoriations, alopecia, salivary staining (lick granulomas), and, with chronicity, hyperpigmentation and lichenification (thickening of the skin). These inflamed and damaged tissues provide an ideal environment for secondary infections, making recurrent superficial pyoderma and Malassezia dermatitis common and significant complications that further amplify the level of pruritus.
While CAD is the most complex allergic syndrome in GSDs, two other hypersensitivities are critical differentials. Flea Allergy Dermatitis (FAD) is an intensely pruritic reaction to proteins in flea saliva. In highly sensitized GSDs, a single flea bite can provoke a severe, widespread reaction. The classic presentation is intense pruritus focused on the caudal half of the body, particularly the lumbosacral region, tail base, perineum, and caudal-medial thighs. Cutaneous Adverse Food Reactions (CAFR), or food allergies, are another important cause of non-seasonal pruritus that can be clinically indistinguishable from CAD. The most common triggers are dietary proteins, with beef and chicken being frequently implicated. The diagnosis of CAFR depends exclusively on the successful execution of a strict dietary elimination trial, as serological and intradermal tests for food allergens are unreliable.
Dietary Factors
Nutrition plays a dual role in the dermatological health of the German Shepherd Dog. It can be a primary source of pathology, as in the case of cutaneous adverse food reactions (CAFR), or it can be harnessed as a powerful therapeutic tool for managing inflammatory skin conditions and supporting barrier function. A complete and balanced diet is the foundation of cutaneous health, providing the essential proteins, fatty acids, vitamins, and minerals required for keratinocyte turnover, immune cell function, and the synthesis of the epidermal lipid barrier.
The definitive diagnosis of CAFR relies solely on the use of a dietary elimination trial, which remains the gold standard diagnostic procedure. This is a critical point, as serological and intradermal tests for food allergens have consistently been shown to lack the accuracy and reliability required for clinical decision-making. The indication for an elimination trial is any case of non-seasonal pruritus, particularly when parasitic causes have been ruled out and the clinical signs are consistent with an allergic etiology. The trial is designed to remove all potential offending dietary antigens and observe for a corresponding resolution of clinical signs. Two primary types of diets are utilized for this purpose. The first is a novel ingredient diet, which consists of a single protein source and a single carbohydrate source that the patient has never been exposed to previously. Success with this approach hinges on obtaining an exhaustive and accurate dietary history from the owner, which can be challenging. Common novel protein sources include venison, rabbit, kangaroo, or alligator. The second, and often preferred, option is a hydrolyzed protein diet. In these prescription formulations, the protein source (e.g., soy, chicken) has been enzymatically broken down into small peptides and amino acids. These fragments are typically too small to be recognized by the immune system and are thus unable to bridge IgE receptors on mast cells to trigger an allergic reaction. Hydrolyzed diets offer the significant advantage of bypassing the need for a perfect dietary history and avoiding potential issues with unrecognized protein cross-reactivity. It is imperative to use a veterinary therapeutic diet for this purpose, as over-the-counter “limited ingredient” diets have been shown to frequently contain contaminating proteins from other food sources due to less stringent manufacturing protocols, which can invalidate the trial results.
The protocol for an elimination trial must be executed with absolute stringency to be valid. The selected diet must be fed exclusively for a minimum period of 8 to 12 weeks. During this time, the dog must not receive any other food items, including treats, table scraps, rawhides, dental chews, or flavored medications (such as chewable heartworm or flea preventatives). Owner compliance is the single most critical factor for success. If a significant reduction in pruritus and clinical lesions is observed by the end of the trial period, a food allergy is strongly suspected. The diagnosis is definitively confirmed by performing a “re-challenge,” where the original diet or individual ingredients are reintroduced. A relapse of clinical signs upon re-challenge provides definitive proof of a CAFR.
Beyond diagnosis, diet can be used therapeutically. The supplementation of omega-3 fatty acids, specifically eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) derived from marine fish oil, is a well-established adjunctive therapy for inflammatory skin conditions like CAD. These fatty acids act as competitive substrates for enzymes involved in the inflammatory cascade, leading to the production of less potent inflammatory mediators compared to those derived from omega-6 fatty acids. This immunomodulatory effect can help reduce the level of pruritus and the required dose of more potent pharmaceuticals. To be effective, omega-3s must be supplemented at therapeutic dosages, which are typically much higher than the maintenance levels found in most commercial dog foods.
Finally, a critical evaluation of raw food diets is warranted. While proponents often claim benefits for skin health and allergies, these assertions are not supported by scientific evidence. Conversely, there are substantial, well-documented risks associated with feeding raw meat-based diets. Numerous studies have demonstrated a high prevalence of contamination with pathogenic bacteria, including Salmonella, Listeria monocytogenes, and toxigenic E. coli. Furthermore, these diets are a significant source of antimicrobial-resistant bacteria, posing a public health risk to both the pet and human members of the household. From an allergy perspective, feeding a protein in its raw state does not alter its allergenic potential; a dog allergic to chicken protein will react whether it is cooked or raw. Given the significant and proven risks of infectious disease and the lack of any scientifically validated benefit, the feeding of raw diets is not recommended in veterinary medicine.
Health Issues that Affect German Shepherds
The skin often serves as a barometer for an animal’s overall health, and in the German Shepherd Dog, cutaneous signs can be the first or most prominent manifestation of a variety of underlying systemic diseases. The breed’s well-documented predisposition to specific endocrine, immune-mediated, and other heritable disorders makes a holistic diagnostic approach essential. The clinical signs of these systemic conditions—such as alopecia, recurrent pyoderma, and changes in skin quality—can mimic primary dermatoses, creating a complex diagnostic challenge. Failure to investigate and identify these underlying problems will invariably lead to therapeutic failure for the presenting skin complaint.
Endocrinopathies are a significant cause of secondary skin disease in GSDs. Hypothyroidism, resulting from insufficient production of thyroid hormones, profoundly affects metabolic rate and skin and hair follicle health. The classic cutaneous signs are typically non-pruritic (unless complicated by secondary infection) and include a bilaterally symmetric truncal alopecia that spares the head and extremities, a dull, dry, and brittle coat that fails to regrow after clipping, and hyperpigmentation of the underlying skin. The skin itself may become thickened, cool to the touch, and in some cases, exhibit a “tragic” facial expression due to myxedema, the deposition of mucin in the dermis. Seborrhea and recurrent superficial pyoderma or Malassezia dermatitis are extremely common sequelae due to altered skin barrier function and local immunity. Conversely, hyperadrenocorticism (Cushing’s disease), characterized by excessive cortisol production, also has profound dermatologic consequences. The catabolic effects of cortisol lead to bilaterally symmetric alopecia, marked thinning of the skin (cutaneous atrophy) which makes abdominal vasculature prominent, and the formation of comedones. A pathognomonic, though not universally present, lesion is calcinosis cutis, which manifests as gritty, mineralized plaques within the dermis. The profound immunosuppressive effects of chronic hypercortisolism render these dogs highly susceptible to severe, recurrent, and often deep pyoderma, as well as generalized demodicosis.
Beyond endocrine disorders, the GSD is defined by inherent immunological vulnerabilities that are primary health issues in their own right. The breed has a well-documented predisposition to Immunoglobulin A (IgA) deficiency. As the principal antibody at mucosal surfaces, secretory IgA is the first line of specific immune defense for the skin, respiratory tract, and gastrointestinal tract. A deficiency in IgA production cripples this defense, predisposing the dog to recurrent bacterial and fungal infections at these sites. This inherent immunodeficiency is considered a key contributing factor to the high prevalence and severity of both GSP and CAD in the breed. Other heritable immune-mediated conditions with prominent skin manifestations are also overrepresented in GSDs. Perianal fistulas, characterized by chronic, suppurative, and ulcerative tracts in the perianal tissues, are now understood to be an immune-mediated disease with parallels to Crohn’s disease in humans. While less common, familial cutaneous vasculopathy is a severe, heritable genodermatosis reported in GSD puppies that causes fever, lethargy, and ischemic necrosis of the extremities, manifesting as crusting and ulceration of the footpads, ear tips, and tail tip. The prevalence of these genetically-linked disorders underscores the critical importance of responsible breeding practices, including the use of available genetic screening tests and the removal of affected individuals from breeding programs, to improve the overall health of the breed.
Parasites and Pests
Ectoparasites are a frequent cause of dermatological disease in all dogs, and their role can range from direct infestation to triggering severe allergic hypersensitivity reactions. In a breed as susceptible to skin inflammation as the German Shepherd Dog, the presence of parasites can significantly exacerbate underlying conditions and complicate the clinical picture. Therefore, the implementation of a rigorous, consistent, and year-round ectoparasite control program is a fundamental and non-negotiable component of managing any GSD with dermatological signs. Parasitic dermatoses are consistently reported as one of the most common diagnostic categories in small animal practice.
Fleas, primarily Ctenocephalides felis, are ubiquitous and play a dual pathogenic role. A heavy infestation can cause significant pruritus, anemia, and discomfort from the bites alone. More importantly in the GSD, fleas are the source of potent salivary antigens that trigger Flea Allergy Dermatitis (FAD) in sensitized individuals. Due to the breed’s hyper-reactive immune system, the response can be severe, making strict flea control paramount. Effective management requires treating not only the dog but also all other in-contact animals and the environment to break the flea life cycle.
Mites are another critical group of parasites. Demodectic mange, caused by an overpopulation of the commensal mite Demodex canis, is a particularly significant disease. These mites reside within hair follicles and are part of the normal cutaneous fauna, kept in check by a competent immune system. The development of generalized demodicosis in an adult dog is therefore a profound clinical marker of an underlying systemic illness or state of immunodeficiency, such as hyperadrenocorticism, hypothyroidism, or neoplasia. The clinical signs include multifocal to diffuse alopecia, erythema, scaling, and comedones. Severe secondary bacterial furunculosis is a common and serious complication, leading to a painful and purulent dermatitis. The diagnosis is made by identifying large numbers of mites on deep skin scrapings or hair plucks (trichograms).
The treatment of generalized demodicosis has been revolutionized by the advent of the isoxazoline class of ectoparasiticides. These drugs, which include fluralaner, afoxolaner, sarolaner, and lotilaner, are administered orally or topically and have demonstrated exceptionally high efficacy and a favorable safety profile. They have largely supplanted older treatment protocols (e.g., amitraz dips, high-dose macrocyclic lactones), which were more cumbersome, had a higher risk of toxicity, and were often less effective. While not all isoxazoline products are specifically licensed for the treatment of demodicosis in every country, their extra-label use for this indication is now considered the standard of care by veterinary dermatologists worldwide. Treatment should be continued until two consecutive negative skin scrapings are obtained one month apart. Sarcoptic mange (Sarcoptes scabiei), an intensely pruritic and contagious mite infestation, is another important differential for any pruritic GSD.
Finally, ticks are important vectors of various systemic pathogens that can have cutaneous manifestations, such as Borrelia burgdorferi (Lyme disease) and Ehrlichia species. While the skin lesions are often non-specific, the presence of tick-borne disease can contribute to systemic illness and immune dysregulation, further complicating the management of a dermatological patient. This reinforces the necessity of using products with proven acaricidal efficacy as part of a comprehensive parasite control strategy.
Table 3: Therapeutic Protocols for Generalized Demodicosis
|
Drug Class |
Active Ingredient |
Brand Name Example(s) |
Administration & Dosing Regimen |
Key Efficacy/Safety Notes |
|
Isoxazolines |
Fluralaner |
Bravecto |
Oral tablet or topical solution; ≥25 mg/kg administered once every 12 weeks. |
Extremely high efficacy (>99% mite reduction) with rapid onset of action. Provides extended duration of protection. Neurological adverse effects (tremors, ataxia, seizures) are rare but have been reported, primarily in animals with a pre-existing history of seizures. |
|
Afoxolaner |
NexGard |
Oral chewable tablet; ≥2.5 mg/kg administered monthly. |
High efficacy. Several formulations are specifically licensed for the treatment of generalized demodicosis. Often combined with milbemycin oxime for broad-spectrum parasite control. |
|
|
Sarolaner |
Simparica |
Oral chewable tablet; 2–4 mg/kg administered monthly. |
High efficacy. Studies have demonstrated non-inferiority or superiority to older treatment modalities. Also available in combination products (e.g., Simparica Trio). |
|
|
Lotilaner |
Credelio |
Oral tablet; ≥20 mg/kg administered monthly. |
High efficacy. Rapid absorption and onset of action. |
|
|
Macrocyclic Lactones |
Imidacloprid / Moxidectin |
Advantage Multi, Advocate |
Topical spot-on solution; Typically applied weekly for demodicosis (extra-label use). |
Effective, but requires more frequent administration and often a longer treatment course compared to isoxazolines. Can be an alternative if isoxazolines are contraindicated. |
Autoimmune Diseases
Autoimmune skin diseases, or immunodermatoses, represent a category of serious and often severe conditions that arise when the host’s immune system erroneously targets and attacks its own cutaneous structures. In these diseases, a breakdown in self-tolerance leads to the production of autoantibodies or autoreactive T-lymphocytes directed against components of the epidermis, basement membrane zone, or other dermal elements. While less common overall than allergic skin diseases, they are overrepresented in certain breeds, including the German Shepherd Dog. These conditions typically require lifelong immunosuppressive therapy for management and carry a guarded prognosis.
The most frequently diagnosed autoimmune skin disease in the dog is Pemphigus Foliaceus (PF). The pathophysiology of PF is a classic example of a type II (antibody-mediated) hypersensitivity reaction. The immune system produces autoantibodies, primarily IgG, that target desmoglein-1, a transmembrane glycoprotein that is a key component of desmosomes in the superficial layers of the epidermis. Desmosomes are responsible for cell-to-cell adhesion between keratinocytes. When autoantibodies bind to desmoglein-1, they disrupt this adhesion, causing the keratinocytes to separate from one another in a process called acantholysis. This loss of cohesion leads to the formation of microscopic clefts within the stratum granulosum or subcorneal layers, which rapidly fill with neutrophils and the detached, rounded keratinocytes (known as acantholytic cells), forming a pustule. While the trigger for this loss of self-tolerance is often unknown (idiopathic), PF has been documented to be induced by certain drugs (e.g., sulfonamides) or may arise in the context of chronic underlying inflammatory or neoplastic disease.
The clinical presentation of PF is characteristic. The primary lesion is a large, flaccid, and fragile pustule, which may be transient and easily missed by the owner. These pustules readily rupture, leaving behind shallow erosions that quickly become covered by prominent, honey-colored crusts and scale. The lesions typically begin symmetrically on the face, particularly the dorsal muzzle (bridge of the nose), nasal planum, and pinnae. The footpads are also a primary site, often showing hyperkeratosis and crusting. From these initial sites, the disease can progress to become generalized, affecting the trunk and limbs. Pruritus and pain are variable but can be significant. Systemic signs such as lethargy, anorexia, and fever may be present in severe, generalized cases.
Diagnosis of PF relies on a combination of cytology and, definitively, histopathology. Cytology, obtained by gently lifting a crust and making an impression smear of the underlying erosion or by aspirating an intact pustule, is a highly valuable in-house test. The pathognomonic finding is the presence of acantholytic keratinocytes—large, round, basophilic epithelial cells with a central nucleus, often found singly or in small clusters (“rafts”)—surrounded by a sea of intact neutrophils and, occasionally, eosinophils. The absence of bacteria differentiates it from bacterial folliculitis. Skin biopsy for histopathology provides the definitive diagnosis. Multiple punch biopsies should be taken from primary lesions (intact pustules) or the edge of fresh erosions. The characteristic histologic finding is a subcorneal or intragranular pustule containing numerous neutrophils, eosinophils, and acantholytic keratinocytes.
Management of PF necessitates systemic immunosuppression. The cornerstone of therapy is the use of high-dose oral glucocorticoids, such as prednisone or prednisolone, to induce remission. Once clinical control is achieved, the dose is slowly tapered over several weeks to months to the lowest possible effective alternate-day dose to minimize side effects. Due to the significant adverse effects associated with long-term, high-dose glucocorticoid use, a second, “steroid-sparing” immunosuppressive agent is typically introduced concurrently. In dogs, azathioprine is a common choice, though it has a slow onset of action (4-6 weeks). Other options include modified cyclosporine, mycophenolate mofetil, or chlorambucil. Adjunctive therapies, such as strict sun avoidance, are also important as UV light can exacerbate lesions.

Pemphigus foliaceous in a german shepherd
Another autoimmune condition seen in GSDs is Discoid Lupus Erythematosus (DLE), which is generally considered a more benign and localized variant of lupus. DLE primarily affects the nasal planum, causing a loss of the normal cobblestone texture, depigmentation, erythema, scaling, erosions, and ulceration. Lesions can be exacerbated by sun exposure. Treatment often involves topical immunomodulators (e.g., tacrolimus), tetracycline/niacinamide combination therapy, and sun avoidance, with systemic glucocorticoids reserved for more severe cases.
Table 4: Immunosuppressive Protocols for Canine Pemphigus Foliaceus
|
Therapy Tier |
Drug(s) |
Induction Dosage |
Maintenance Dosage |
Key Monitoring & Side Effects |
|
Tier 1 (Induction) |
Prednisone / Prednisolone |
2.0–4.0 mg/kg/day PO, often divided BID initially. |
Taper slowly over 8-12 weeks to the lowest effective alternate-day (EOD) dose, aiming for <1.0 mg/kg EOD. |
Polyuria/polydipsia (PU/PD), polyphagia, muscle wasting, panting, GI ulceration, iatrogenic hyperadrenocorticism. Monitor CBC, chemistry panel, and urinalysis periodically. |
|
Tier 2 (Adjunct) |
Azathioprine (DOGS ONLY) |
2.0 mg/kg/day PO for 1-2 weeks, then reduce to EOD. |
1.0–2.0 mg/kg EOD. |
Delayed onset of action (4–6 weeks). Potential for severe bone marrow suppression and hepatotoxicity. Requires frequent CBC and chemistry monitoring, especially during initiation. DO NOT USE IN CATS. |
|
Tier 2 (Adjunct) |
Modified Cyclosporine |
5–10 mg/kg/day PO. |
Taper to lowest effective frequency (EOD or twice weekly) once remission is achieved. |
Common: GI upset (vomiting, diarrhea), which often resolves. Less common: gingival hyperplasia, hirsutism, papillomatosis. Therapeutic drug monitoring may be useful initially. |
|
Tier 3 (Refractory) |
Mycophenolate Mofetil |
10–20 mg/kg PO BID. |
Taper to lowest effective dose. |
Primarily GI side effects (diarrhea). Used in cases refractory to standard therapies. |
|
Tier 3 (Refractory) |
Chlorambucil |
0.1–0.2 mg/kg/day PO. |
Taper to EOD. |
Generally well-tolerated. Myelosuppression is the primary concern; requires periodic CBC monitoring. Often used in combination with glucocorticoids. |
Environmental Factors
The development and severity of dermatological disease in a genetically susceptible individual like the German Shepherd Dog is profoundly influenced by the sum of its environmental exposures, a concept known as the “exposome.” For a GSD with an inherently compromised skin barrier and a hyper-reactive immune system, environmental factors often act as the critical trigger that initiates the cascade of inflammation and pushes the dog from a subclinical state into overt clinical disease. Management of the dog’s immediate environment is therefore a crucial component of a holistic therapeutic strategy.
The most significant environmental influence in GSD skin disease is exposure to the aeroallergens that drive canine atopic dermatitis. The indoor environment is a particularly potent reservoir for these triggers, providing year-round exposure. House dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus) are among the most important perennial allergens worldwide. These microscopic arachnids thrive in bedding, carpets, and upholstery. Storage mites (e.g., Tyrophagus putrescentiae, Acarus siro) can contaminate dry pet foods that are not stored in airtight containers. Outdoor allergens, which can be seasonal, include pollens from trees, grasses, and weeds, as well as various mold spores. These microscopic particles readily penetrate the defective epidermal barrier of an atopic GSD, initiating the allergic cascade.
Beyond specific allergens, a range of other environmental factors can act as irritants, further compromising skin barrier function and exacerbating inflammation. Low ambient humidity, common in heated homes during winter, can increase transepidermal water loss and lead to xerosis (dry skin), which itself can be pruritic. The use of harsh or inappropriate shampoos can strip the skin of its natural protective lipids, worsening barrier dysfunction. Direct contact with chemicals in cleaning products, carpets, or lawn treatments can also cause irritant contact dermatitis. Furthermore, human dander, while a less common allergen, can also contribute to the overall antigenic load in the home environment.
Consequently, environmental management strategies aimed at reducing the patient’s exposure to these triggers are a vital part of multimodal therapy. Frequent bathing (once or twice weekly) with a mild, hypoallergenic, or medicated shampoo serves to physically remove allergens, microbes, and crust from the skin and coat, providing direct relief and reducing the inflammatory stimulus. In the home, the use of high-efficiency particulate air (HEPA) filters can significantly reduce the concentration of airborne allergens. Regular and thorough vacuuming of carpets and furniture, preferably with a vacuum equipped with a HEPA filter, is also beneficial. Washing the dog’s bedding frequently in hot water helps to kill dust mites. Using allergen-impermeable covers on the dog’s bed can prevent mite colonization. Interestingly, some epidemiological studies support a version of the “hygiene hypothesis,” suggesting that early-life exposure to more diverse environments, such as rural settings or households with other animals, may be protective against the later development of atopic disease, possibly by promoting a more balanced maturation of the immune system.
Veterinary Care and Diagnosis
The successful management of skin disease in the German Shepherd Dog is predicated on a systematic and thorough diagnostic approach. Given the breed’s propensity for complex, multifactorial, and overlapping conditions, arriving at a definitive diagnosis before committing to long-term or potent therapies is paramount. The diagnostic journey begins with the foundational elements of a comprehensive patient history and a detailed physical and dermatological examination. Key historical points include the age of onset, seasonality of signs, description and quantification of pruritus, diet, ectoparasite control, response to previous therapies, and the presence of clinical signs in other household pets or humans.
Following the initial assessment, a suite of foundational, minimally invasive, and cost-effective in-clinic diagnostic tests should be performed. Cutaneous cytology is arguably the single most valuable tool in the dermatologic workup. The technique for sample collection is tailored to the lesion type: direct impression smears for moist or exudative lesions, acetate tape preparations for dry, scaly, or difficult-to-access areas (like interdigital spaces), and fine-needle aspiration for nodules or pustules. Microscopic examination under oil immersion provides immediate, invaluable information. The clinician can identify the nature of the inflammatory infiltrate (e.g., neutrophilic, eosinophilic, pyogranulomatous), the presence and type of infectious organisms (cocci, rods, Malassezia yeast), and characteristic cell types such as acantholytic keratinocytes, which are suggestive of Pemphigus Foliaceus. The identification of intracellular bacteria within neutrophils is the definitive cytological confirmation of a true bacterial infection (pyoderma). Skin scrapings are essential for the diagnosis of parasitic disease. Deep skin scrapings, performed by pinching the skin and scraping until capillary oozing is observed, are the standard for detecting Demodex mites within hair follicles. Multiple sites should be sampled. Superficial scrapings are used to look for surface-dwelling mites like Sarcoptes scabiei or Cheyletiella. A trichogram, or microscopic examination of plucked hairs, is a useful adjunctive test. It can reveal Demodex mites, especially from locations that are difficult to scrape (e.g., periorbital, interdigital), and can be used to assess hair shafts for evidence of dermatophytosis (fungal hyphae/arthroconidia) or structural abnormalities seen in follicular dysplasias.
For cases that are diagnostically challenging, non-responsive to appropriate therapy, or where neoplasia or autoimmune disease is suspected, skin biopsy for histopathology is the gold standard. Proper technique is critical for obtaining a diagnostic sample. Multiple (3-4) samples should be collected using a 6-mm or 8-mm punch biopsy tool. The site should be clipped but never surgically scrubbed, as important diagnostic clues (crusts, scale) may be located on the surface. Primary lesions (pustules, papules, vesicles) should be selected whenever possible. The tissue plug should be handled gently to avoid crush artifact and placed immediately into 10% buffered formalin. Providing the pathologist with a detailed clinical history, description of lesions, and a list of differential diagnoses is crucial for an accurate histopathological interpretation.
When canine atopic dermatitis is diagnosed based on clinical criteria, allergy testing may be performed. It is critical to understand that these tests do not diagnose atopy; rather, they are used to identify the specific environmental allergens to which the patient is sensitized, in order to formulate patient-specific allergen immunotherapy (ASIT). The two primary methods are intradermal allergy testing (IDAT) and serum IgE testing. IDAT, considered the gold standard by many dermatologists for its specificity, involves injecting small amounts of various allergens into the skin and observing for a local wheal-and-flare reaction. Its disadvantages include the need for patient sedation, shaving of the lateral thorax, and a prolonged withdrawal period from glucocorticoids and antihistamines. Serum IgE testing is more convenient, requiring only a blood sample with no medication withdrawal. However, this method is known to have a higher rate of false-positive results, and the clinical relevance of some positive results is debatable, as clinically normal dogs can have circulating allergen-specific IgE. Emerging research into pathogenic versus non-pathogenic IgE isotypes may lead to more specific serum tests in the future. Currently, some specialists advocate for performing both tests to maximize the identification of clinically relevant allergens for inclusion in an ASIT protocol.
Table 5: Comparative Overview of Dermatological Diagnostic Techniques
|
Diagnostic Test |
Primary Indication |
Sample Required |
Key Finding |
Clinical Utility / Limitations |
|
Cutaneous Cytology |
Infection, inflammation, initial screening for Pemphigus Foliaceus. |
Impression smear, acetate tape prep, fine-needle aspirate. |
Bacteria (cocci, rods), yeast (Malassezia), inflammatory cells (neutrophils, eosinophils), acantholytic keratinocytes. |
High diagnostic yield, rapid, inexpensive, performed in-house. Interpretation is a learned skill. |
|
Deep Skin Scraping |
Demodicosis (Demodex canis). |
Full-thickness epidermal/dermal sample in mineral oil. |
Demodex mites (adults, eggs, larvae). |
Gold standard for diagnosing demodicosis. Can produce false negatives if mite population is low or sampling is inadequate. |
|
Skin Biopsy & Histopathology |
Suspected neoplasia, autoimmune disease, vesicular diseases, or any condition non-responsive to therapy. |
6-8mm full-thickness skin punch biopsy fixed in 10% buffered formalin. |
Assessment of tissue architecture, specific inflammatory patterns, cell morphology, and presence of organisms. |
Definitive diagnosis for many complex diseases. Essential for prognostication. Requires pathologist interpretation; results take several days. |
|
Intradermal Allergy Test (IDAT) |
To identify environmental allergens for formulating Allergen-Specific Immunotherapy (ASIT) in a confirmed atopic dog. |
N/A (procedure). |
A visible and palpable wheal and flare reaction at the site of allergen injection. |
Considered the gold standard for specificity in identifying causative allergens. Invasive, requires sedation and prolonged drug withdrawal. |
|
Serum IgE Test |
To identify environmental allergens for formulating ASIT in a confirmed atopic dog. |
Serum sample. |
Elevated levels of circulating allergen-specific IgE. |
Convenient (simple blood draw), no drug withdrawal required. Higher rate of false positives; clinical relevance of some positive results can be questionable. |
Conclusion
The dermatological constitution of the German Shepherd Dog is uniquely precarious, defined by a confluence of heritable vulnerabilities that create a predisposition to chronic and complex skin disease. This review has synthesized the evidence demonstrating that the breed’s most common and challenging skin ailments are not discrete events but are manifestations of a fundamental syndromic state. This state is characterized by a triad of defects: a genetically programmed, structurally deficient epidermal barrier, exemplified by mutations in genes like PKP2; a functionally impaired mucosal immune system, highlighted by a predisposition to IgA deficiency; and a systemically hyper-reactive immune response that readily mounts an allergic, Th2-polarized inflammatory cascade. This triad mechanistically explains the breed’s profound susceptibility to the vicious cycle of allergy, inflammation, barrier breakdown, and the deep-seated secondary infections that are the hallmark of its clinical presentation. Successful management, therefore, demands a paradigm shift from simply treating symptoms to addressing these foundational liabilities through a persistent, multimodal strategy.
Looking forward, the advancement of veterinary dermatology for the German Shepherd Dog hinges on targeted research that moves beyond characterizing disease to preventing it. Several avenues of investigation hold particular promise. First, genetic and epigenetic research must progress from identifying risk loci to whole-genome sequencing and functional genomics. This will allow for the identification of a more complete network of causative genes, regulatory elements, and, crucially, the epigenetic modifications through which environmental factors influence the expression of this genetic risk. Second, and perhaps most importantly, is the imperative to develop strategies for the primary prevention of canine atopic dermatitis. Drawing from promising work in human pediatrics, prospective, longitudinal studies in high-risk GSD puppies are urgently needed. Such trials could rigorously evaluate the efficacy of early-life interventions aimed at bolstering the skin barrier (e.g., daily application of topical lipid emollients) and beneficially modulating the maturing immune system (e.g., administration of specific probiotic strains, controlled environmental exposures). Third, for established diseases like German Shepherd Pyoderma, research must focus on elucidating the specific cellular and molecular defects in the breed’s T-cell response. This knowledge could pave the way for targeted immunomodulatory therapies—such as monoclonal antibodies or small molecule inhibitors—that can correct the precise immune imbalance, offering a more refined alternative to the current reliance on broad-spectrum antibiotics and immunosuppressants. Finally, comprehensive studies of the cutaneous and gut microbiome in healthy versus affected GSDs are critical. Characterizing the dysbiosis associated with CAD and GSP could unlock novel therapeutic avenues such as bacteriotherapy, phage therapy, or fecal microbiota transplantation.
For the practicing clinician, the ultimate message is that managing skin disease in a German Shepherd Dog requires a holistic, proactive, and deeply informed clinical mindset. Success is rarely achieved through short-term, reactive interventions. Instead, it is contingent upon a sustained, multifaceted approach that simultaneously controls underlying allergies, actively works to restore and protect the epidermal barrier, diligently prevents and manages secondary infections, and provides the owner with the education and tools for lifelong preventative care. By understanding the fundamental biology of why this noble breed is so vulnerable, veterinarians can more effectively break the cycle of disease and substantially improve the quality of life for these patients.
Références
- Bell, S. M., Evans, J. M., Evans, K. M., Tsai, K. L., Noorai, R. E., Famula, T. R., & Clark, L. A. (2022). Congenital idiopathic megaesophagus in the German shepherd dog is a sex-differentiated trait and is associated with an intronic variable number tandem repeat in Melanin-Concentrating Hormone Receptor 2. PLoS Genetics, 18(3), e1010044.
- Denerolle, P., et al. (2008). German Shepherd dog pyoderma: A prospective study of 23 cases. Veterinary Dermatology, 9(4), 243-248.
- Dunstan, R. W., et al. (1997). A hereditary cutaneous vasculopathy in German shepherd dogs. Veterinary Pathology, 34(5), 501.
- Fondati, A., Fondevila, M. D., Minghelli, A., Romano, E., & Varazzani, B. (1998). Familial cutaneous vasculopathy and demodicosis in a German shepherd dog. Journal of Small Animal Practice, 39(3), 137-9.
- Hillier, A., et al. (2024). International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial skin infections in dogs. Veterinary Dermatology.
- Khoshnegah, J., Azari, M., & Alimolaei, M. (2015). Survey of canine dermatological conditions in a referral small animal practice, Mashhad, Iran. Journal of the South African Veterinary Association, 86(1), 1198.
- Kim, M., et al. (2024). Prevalence of Bacterial Pathogens Isolated from Canines with Pyoderma and Otitis Externa in Korea: A Systematic Review and Meta-Analysis. Veterinary Sciences, 11(12), 656.
- Lübke-Becker, A., et al. (2024). Prevalence and Antimicrobial Resistance of Staphylococcus pseudintermedius in Dogs and Cats: A Retrospective Study from 2019 to 2021 in Germany. Veterinary Sciences, 13(7), 660.
- Marsella, R., et al. (2023). Oclacitinib 10 years later: lessons learned and directions for the future. Journal of the American Veterinary Medical Association, 261(S1), S36–S47.
- Mueller, R. S., et al. (1999). Comparison of intradermal testing and serum testing for allergen-specific IgE using monoclonal IgE antibodies in 84 atopic dogs. Veterinary Dermatology, 10(3), 179-184.
- Olivry, T., et al. (2015). Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). Veterinary Dermatology, 26(4), 234-e53.
- Proverbio, D., et al. (2014). A Retrospective Study of Dermatological Lesions in a Population of 100 Dogs with Leishmaniasis. The Scientific World Journal, 2014, 934269.
- Pu, J., et al. (2024). Canine Atopic Dermatitis: An Update on the Pathogenesis, Diagnosis, and Treatment. Veterinary Sciences, 11(2), 107.
- Silvestre, G. S., Gütschow, M. E. R. P., Meira, J., Custódio, C. S., Motheo, T. F., & Pereira, M. L. (2021). Prevalence of dermatopathies in dogs and cats in the highland of Santa Catarina State, Brazil. Acta Veterinaria Brasilica, 15(3), 220-224.
- Standl, M., Budu-Aggrey, A., Johnston, L. J., et al. (2025). Gene–Environment Interaction Affects Risk of Atopic Eczema: Population and In Vitro Studies. Allergy.
- Tengvall, K., Kierczak, M., Bergvall, K., Olsson, M., Frankowiack, M., Farias, F. H. G., Pielberg, G., Carlborg, Ö., Leeb, T., Andersson, G., Hammarström, L., Hedhammar, Å., & Lindblad-Toh, K. (2013). Genome-wide analysis in German shepherd dogs reveals association of a locus on CFA 27 with atopic dermatitis. PLoS Genetics, 9(5), e1003475.
- Tsai, K. L., Noorai, R. E., Starr-Moss, A. N., Quignon, P., Rinz, C. J., Ostrander, E. A., … Clark, L. A. (2012). Genome-wide association studies for multiple diseases of the German Shepherd Dog. Mammalian Genome, 23(1–2), 203–211.
- White, S. D., et al. (1997). German Shepherd Dog Pyoderma: A Prospective Study of 12 Dogs. Journal of the American Animal Hospital Association, 33(4).
- Willemse, T. (1989). German shepherd dog pyoderma: a genetic disorder. The Veterinary Quarterly, 11(3), 161-4.
- Zahri, A., Bouslikhane, M., El Mazini, S., Lemrani, M., El Berbri, I., Abouelkaram, M. A., Balenghien, T., & Bourquia, M. (2024). Survey on Dermatological Disorders of Dogs during 2020-2022 in Rabat, Morocco. World Veterinary Journal, 14(3), 449-460.
Search terms
intense itching, secondary skin infections, hair loss, german shepherd skin issues, inflamed skin, flaky skin, environmental allergies, german shepherd skin, itchy skin, contact allergies, yeast infections, skin allergies, insect bites, skin irritation, flea infestations, scaly skin, dog suffers, external parasites, skin disorder, anal sac disease, genetic disease, allergy medications, pet owners, elbow dysplasia, popular dog breed, topical medications, underlying disease, blood tests, oral medications, intense itchiness, excessive licking, red skin, excessive scratching, hormonal imbalances, treatment options, food, allergies, german, shepherds, cutaneous, adverse, food, reaction, develop, food, allergies, skin, infection, hot, spots, essential, fatty, acids, dog’s, skin, skin, condition, dog, food, skin, issue, food, hypersensitivity, developing, allergies, flea, bites, dog’s, allergies