Since Wittich’s first publication in 1941 reporting the successful treatment of a dog’s allergy using ASIT (Allergen-Specific Immunotherapy), significant progress has been made. While numerous human studies extensively document ASIT’s efficacy in various allergic diseases, particularly allergic rhinitis and asthma, data regarding canine atopic dermatitis (CAD) remain, paradoxically, fragmented.
Current Knowledge on Canine ASIT
While some question ASIT’s usefulness in human atopic dermatitis, this controversy seemingly never emerged in veterinary medicine, likely due to encouraging early reports concerning CAD. The lack of controversy in veterinary medicine could also be attributed to factors such as the limited number of rigorous clinical trials conducted in dogs, the complexity of assessing ASIT’s efficacy in the context of CAD, and the variability of therapeutic protocols used across different studies. These factors make direct comparison of results difficult, thus limiting overall conclusions on the efficacy of canine ASIT.
Studies, including a placebo-controlled, blinded study of 51 dogs, have demonstrated response rates (generally defined as the proportion of dogs exhibiting at least a 50% improvement in clinical signs) in the range of 60% to 70%. However, it is crucial to note that even in positive cases, a significant percentage of dogs (up to 65% in one study) required complementary drug treatment, questioning the owners’ ability to objectively judge ASIT’s efficacy. Current guidelines for CAD treatment classify the quality of published evidence regarding ASIT’s efficacy as “limited.” This limitation in evidence is due to several factors, including the small sample size of many studies, the lack of protocol standardization, and the difficulty of obtaining objective measures of ASIT’s efficacy in dogs. It is important to emphasize that the assessment of ASIT’s efficacy often relies on subjective owner assessments, which can introduce bias into the results. More objective evaluation methods, such as the use of biomarkers or validated clinical scores, are needed to improve the quality of available evidence.
Interpretation of canine ASIT study results is often complicated by the variability of protocols used. Differences in dosage, allergen extract composition, injection frequency, concomitant treatments, and methods of evaluating results make direct comparison of results obtained in different studies difficult. The heterogeneity of dog populations included in the studies constitutes another factor of complexity. Variability in breeds, ages, CAD severities, and medical histories of dogs can influence the response to ASIT, making data analysis more complex. To improve data interpretation, future studies should focus on protocol standardization, homogeneity of study populations, and the use of more objective efficacy measures.
Comparison with Human ASIT: Although ASIT is used to treat various allergies in humans, its efficacy and mechanisms of action are not always fully understood and vary depending on the type of allergy and the individual. Controversy exists regarding the usefulness of ASIT in human atopic dermatitis, with some studies questioning its efficacy, while others show positive results. This nuance reflects the complexity of individual immune responses and the heterogeneity of atopic dermatitis. Comparison with human ASIT can identify research avenues for canine ASIT, but it is important to qualify these comparisons due to physiological and immunological differences between species.
Mechanisms of Action: The Known and the Unknown
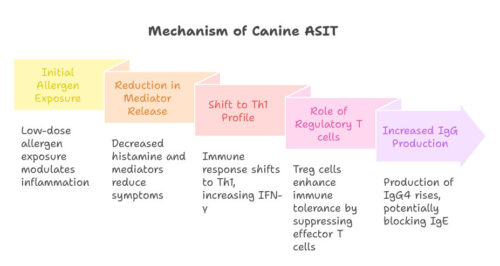
The precise mechanism of action of canine ASIT remains incompletely elucidated. Nevertheless, a parallel with human mechanisms is plausible. In humans, an initial reduction in the activity of effector cells (eosinophils, basophils, mast cells) is observed, followed by a long-term immunological shift from a Th2 (helper 2) cell response to a Th1 (helper 1) cell response and the development of immunological tolerance. This complex, multifactorial process involves different stages and cell populations.
- Initial Phase: Exposure to allergens in low doses causes modulation of the immediate inflammatory response. This results in a reduction in the release of histamine and other inflammatory mediators by mast cells and basophils, reducing immediate allergy symptoms. Mast cell degranulation, a key process in the immediate allergic response, is inhibited or attenuated.
- Late Phase: The immune response gradually shifts towards a Th1 profile, with an increase in Th1 helper lymphocytes that produce pro-inflammatory cytokines such as interferon-gamma (IFN-γ) and a decrease in Th2 helper lymphocytes and associated cytokines (IL-4, IL-5, IL-13). This Th1/Th2 shift contributes to the regulation of the long-term inflammatory response.
- Role of Regulatory T cells (Treg): Treg cells, with markers such as FOXP3, play a crucial role in the immune tolerance acquired by ASIT. These cells suppress the activity of effector T lymphocytes, contributing to the alleviation of the allergic inflammatory reaction. Increased number and activity of Treg cells are correlated with a better response to ASIT.
- IgG Production: The production of allergen-specific immunoglobulins G (IgG), notably IgG4, increases after prolonged treatment. These blocking IgG antibodies could reduce the binding of IgE to allergens, thus decreasing their ability to trigger an inflammatory response. However, studies remain uncertain about the actual impact of IgG on the reduction of the allergic response.
These changes are accompanied by an increase in cytokines such as transforming growth factor beta (TGFβ) and interleukin (IL)-10, anti-inflammatory cytokines playing a role in maintaining immune tolerance. This results in an increase in allergen-specific immunoglobulins (IgG), particularly IgG4, and, with prolonged treatment, a decrease in allergen-specific IgE. In dogs, although knowledge is less extensive, a shift towards a Th1 response, an increase in IgG levels, the appearance of more Treg cells, and increases in IL-10 levels have all been demonstrated, thus establishing parallels with human ASIT. However, studies in dogs are less numerous and less in-depth than in humans, and exploration of the mechanisms of action of canine ASIT requires further research for a more complete understanding. The use of animal models (mice, for example) can help elucidate certain mechanisms, but extrapolation to the canine species must be done cautiously.
Sublingual administration involves an additional effect through oromucosal dendritic cells. In humans, these cells play an important role in the induction of oral tolerance, an immunological process by which the immune system is programmed not to react to substances introduced into the mouth (e.g., food). The precise role of these cells in canine ASIT remains to be explored.
Current Approaches to Canine ASIT: Efficacy and Areas for Improvement
Current ASIT approaches for CAD rely on two main administration routes: subcutaneous (SCIT) and sublingual (SLIT). SCIT, practiced for decades, uses aqueous allergen extracts preserved with phenol in North America and alum-precipitated allergen extracts in Europe. The use of aluminum-based adjuvants, despite the advantage of less frequent injections, raises growing concerns about its potential long-term adverse effects, related to chronic exposure to aluminum. In-depth studies on the long-term toxicity of aluminum are needed to fully assess the implications of its use in vaccines and allergy treatments. Alternatives to aluminum-based adjuvants are currently being studied, including more biocompatible and less toxic adjuvants.
SLIT, more prevalent in humans, has appeared more recently in veterinary medicine and is the subject of controversy in humans regarding the reliability of many older studies with highly variable protocols. Recent analyses and positions by the World Allergy Organization conclude that it is effective and has a favorable safety profile compared to SCIT. However, data on canine SLIT are still limited and require further studies to validate its efficacy and safety in this species. Studies in humans suggest that SLIT could be a more practical and safer approach than SCIT. However, the variability of protocols used in human studies makes it difficult to determine optimal protocols for canine SLIT, requiring future studies to validate its use in dogs. Similarly, comparative studies between SCIT and SLIT in CAD are essential to evaluate their relative efficacy and the benefits and drawbacks of each method.
Important considerations regarding the quality of the extracts used affect efficacy. Allergen extracts for veterinary use were licensed by the United States Department of Agriculture (USDA) decades ago, based on criteria of safety, purity, sterility, and manufacturing consistency, and not on rigorous long-term efficacy studies. This situation highlights the need for new regulations and stricter standards to ensure the quality and efficacy of allergen extracts used in veterinary medicine.
The scientific literature on canine ASIT suffers from a lack of standardization of protocols, with great variability in dosage regimens, allergenic extracts, their composition and potency between manufacturers, administration programs, concomitant treatments, and methods of mixing extracts. This lack of standardization limits the comparability of studies and the interpretation of results. The absence of standardized protocols makes it difficult to assess the true efficacy of ASIT and to determine the optimal parameters for the treatment of CAD.
Details on Different Approaches:
- SCIT (Subcutaneous): The allergen is injected under the skin. Several approaches exist, with a gradual increase in doses (induction) and a long-term maintenance schedule. Extracts can be aqueous (North America) or alum-precipitated (Europe) for slower release. The use of alum raises questions about the long-term toxicity of aluminum, requiring studies and a benefit-risk assessment.
- SLIT (Sublingual): The allergen is administered under the tongue, a less invasive approach potentially better for owner compliance. However, more studies and better standardization are needed for its calibration in veterinary clinics.
- RIT (Rush Immunotherapy): Rapid administration of increasing doses of allergen over a short period, requiring close monitoring. Increased risks of adverse effects exist, but it may be more convenient for some dogs.
Improving the Standardization of Canine ASIT
Establishing the major allergenic epitopes in dogs, particularly for common allergens, would allow for standardization of extracts, uniform dosage, production of recombinant allergens, and the use of peptide immunotherapy. Identification of major allergenic epitopes in dogs allows for the creation of more specific and purified extracts. These standardized extracts allow for better reproducibility of results between different studies, thus improving the reliability of the data. In addition, knowledge of epitopes allows for the development of recombinant allergens, synthesized in the laboratory, which allows for the production of purer and more homogeneous allergen preparations.
Allergen dosage is fundamental. In humans, the dose and injection interval affect efficacy. These parameters have not been sufficiently studied in dogs. Dose-response studies are crucial to determine the optimal allergen dose that maximizes efficacy while minimizing adverse effects. The identification of predictive biomarkers of response to ASIT could also allow for personalized treatment based on the individual dog’s response.
Similarly, rush immunotherapy (RIT) offers advantages in terms of the number of home injections, but also increased safety risks and higher costs due to the required hospitalization. Rush immunotherapy (RIT) is an approach that allows for faster administration of allergen doses. This can reduce treatment duration and improve owner compliance, but increases the risk of adverse effects. To optimize the use of RIT, it is necessary to better document the safety and efficacy of this method, and possibly design less intensive protocols to reduce risks while preserving efficacy. Comparative studies comparing RIT with conventional immunotherapy are needed.
Optimization of Canine ASIT Protocols
Homogenization of canine ASIT protocols represents a major challenge. The optimal number of extracts in a mixture remains to be defined, as does how to manage extracts containing proteases, such as molds. Standardization of protocols requires a multifactorial approach. It is necessary to define the optimal treatment parameters, including:
- Number and type of allergens: Determine the number of allergens to include in the mixture. Studies suggest that a single dominant allergen may be sufficient, even in cases of polysensitization, although this approach is less common in North America than in Europe.
- Management of proteases: Molds contain proteases that can degrade other allergens. This can compromise the efficacy of the allergen mixture. This issue requires further study.
- Methods for selecting allergens: Use intradermal tests, serological tests, or a combination of both. The limitations of each method must be taken into account. Additional research is needed to determine the optimal method for selecting allergens for canine ASIT.
- Formulation of the allergen mixture: More studies are needed to determine the best approach, which will allow for improved reproducibility of studies.
Official guidelines recommend not mixing mold and pollen extracts in the same vial due to the degradation of pollen allergens by fungal proteases during storage. Similar, but less conclusive, data exist for dogs. These recommendations highlight the need to better understand the interactions between different allergens in the mixture. It is important to take into account potential enzymatic degradations at the level of the different components of the mixture and thus to determine the optimal formulation in order to guarantee long-term efficacy.
Biomarkers of Therapeutic Success and New Approaches
The use of biomarkers for the objective evaluation of the success of canine ASIT is essential to improve clinical studies, overcome the lack of reproducible data, and overcome the significant placebo effect. Although increases in total serum IgG1 concentrations, specific IgG responses to immunotherapy for house dust mites, and changes in various levels of Treg cells have been observed in dogs, these findings have not been sufficiently investigated to serve as a means of objective and rapid assessment of ASIT success. The identification of reliable biomarkers would allow for better assessment of treatment efficacy and better prediction of individual response to ASIT.
Potential biomarkers to explore include:
- Serum IgG concentrations (especially IgG4): Increased allergen-specific IgG could correspond to clinical improvement. Measurement of IgG4 is particularly promising, as this IgG subclass is particularly linked to tolerance induction.
- Regulatory T cells (Treg): The number and activity of Treg cells are key indicators of immune tolerance. Measurement of circulating Treg cells or those in tissues could be useful for monitoring the response to ASIT.
- Anti-inflammatory cytokines (IL-10, TGF-β): Increased levels of these cytokines could indicate a decrease in allergic inflammation.
- Effector cell activity (eosinophils, basophils, mast cells): Decreased activity could be correlated with a better clinical response.
- Objective measurements of pruritus: For example, the use of accelerometers to quantify scratching activity.
The use of combined biomarkers could provide a more complete picture of the response to ASIT, thus improving the interpretation of results from clinical studies.
New Therapeutic Approaches
The development of modified allergenic preparations (allergoids, recombinant allergens or peptides) and the improvement of allergen efficacy via adjuvants (IL-10 inducers, encapsulation in virus-like particles (VLPs) or, in the case of SLIT, mucoadhesive polymers) represent promising avenues of progress. These modified preparations offer several advantages:
- Allergoids: Chemically modified allergens to reduce their ability to trigger an immediate allergic reaction, while retaining their immunogenic capacity. This would reduce the risk of adverse effects.
- Recombinant allergens: Allergens produced in the laboratory, offering superior purity and homogeneity to natural extracts.
- Allergenic peptides: Small fragments of allergens containing epitopes specific to T lymphocytes, minimizing the risk of anaphylaxis.
The addition of immunomodulators (CpG oligodeoxynucleotides, specific monoclonal antibodies) could direct the immune response towards specific tolerance while moderating the “cytokine storm” in active inflammation. These immunomodulators allow manipulation of the immune response, promoting anti-inflammatory responses and immune tolerance. CpG ODN stimulate innate immunity by activating dendritic cells and NK cells, and monoclonal antibodies can target specific pro-inflammatory cytokines.
Studies conducted on alternative routes of administration (epicutaneous or intralymphatic immunotherapy) are promising. Epicutaneous immunotherapy applied topically to the skin could induce local and systemic tolerance to allergens. Intralymphatic immunotherapy, which involves injecting the allergen directly into the lymph nodes, could optimize contact with the immune cells involved in the development of tolerance. Although studies on intralymphatic immunotherapy in dogs exist, further research is needed to refine optimal protocols and assess its long-term efficacy and safety. In addition, intralymphatic immunotherapy could be less burdensome for the owner compared to classic subcutaneous immunotherapy, which would improve therapeutic compliance.
Furthermore, advances in immunotherapy for human food allergies, including the use of molecular diagnostics and alternative administration methods, could inspire innovative approaches for CAD. Optimizing the diagnosis of food allergies in dogs is an important area of research. More precise molecular diagnostic methods could identify the specific food allergens responsible for the reactions. New administration methods, such as oral treatments, could improve dogs’ tolerance to allergenic proteins. Advances made in humans could be a source of inspiration for adapting these approaches in the context of CAD.
ASIT versus New Treatments
Finally, it is important to remember that, given the efficacy of new treatments such as ciclosporin, oclacitinib, or anti-IL-31 monoclonal antibodies, the place of ASIT in the multimodal treatment of canine allergies requires constant reflection. Nevertheless, ASIT remains the only therapy capable of modifying or reversing at least part of the pathogenesis and offering the prospect of a definitive cure. It is therefore essential that ASIT be considered, not as a last resort treatment, but as a feasible therapeutic option as a first intention. ASIT can be a therapeutic option of choice as a first intention, particularly in dogs that do not respond well to conventional drug treatments, or in those that present adverse effects to these drugs. The combination of ASIT with other treatments can improve the overall efficacy of CAD treatment. It is important to consider the safety profile and relative efficacy of the different treatments available.
Comparison with alternative treatments: Ciclosporin, oclacitinib, and anti-IL-31 monoclonal antibodies are effective treatments for relieving CAD symptoms, but they do not modify the pathogenesis of the disease and their long-term administration is necessary. ASIT, on the other hand, targets the root cause of the allergy, modifying the dog’s immune response. Although ASIT may take time to produce an effect, it offers the prospect of complete cure, as opposed to symptomatic treatments. The combined use of ASIT and symptomatic treatments is a possible approach.
Conclusion and Perspectives
In conclusion, although ASIT is a promising therapeutic option for canine atopic dermatitis, its efficacy remains to be formally demonstrated by rigorous and better standardized clinical studies. The mechanisms of action remain enigmatic, and further avenues of investigation are needed. More numerous and rigorous studies are essential to fill the current gaps in knowledge. It is necessary to better understand the mechanisms of action, determine the optimal treatment parameters, and identify reliable biomarkers to evaluate efficacy and predict response.
Future studies should focus on:
- Precise identification of canine allergens: Development of more precise and standardized diagnostic tests to identify the allergens involved.
- Optimization of administration protocols: Standardization of protocols and comparison of different administration methods (SCIT, SLIT, RIT, ILIT). Rigorous comparative studies are needed.
- Identification of predictive biomarkers of therapeutic success: Determine markers that allow prediction of treatment response before administration. This will allow for personalized treatment.
- Exploration of modified allergenic preparations: Test recombinant allergens, peptides, and allergoids. These purer formulations could improve safety and efficacy.
- Immunomodulatory strategies: Test the use of immunomodulators to influence the immune response and optimize tolerance.
- Long-term studies: Evaluate the long-term effects of different protocols to better understand the durability of treatment efficacy.
ASIT could thus become a first-line treatment in the management of CAD, instead of remaining confined to a second-line option. Improved protocols, standardization, and the development of new diagnostic and therapeutic tools will make ASIT a tool of choice in the treatment of canine atopic dermatitis.
FAQs
Q1: What are the limitations of intradermal and serological tests for the diagnosis of CAD?
Intradermal tests (IDT) and serological tests for the determination of allergen-specific IgE (ASIS) have limitations. IDTs do not measure the sensitivity of all allergic pathways, and false positive reactions in non-allergic dogs are described. False positive reactions are more numerous for higher concentrations, and positive results may be related to non-specific IgE sensitivity (cross-reactions with other allergens). ASIS only measure circulating allergen-specific IgE and do not take other allergic pathways into account, and false positive reactions have also been observed. In addition, both tests lack standardization, and false positive and false negative results are possible. Sensitivity can vary from one laboratory to another.
It is estimated that between 10% and 30% of dogs with clinically confirmed CAD may have a negative IDT, due to various factors such as technique, allergen concentration, drug interferences, intrinsic host factors, or an incorrect choice of allergens. The limitations of intradermal tests also include the need for specific dog preparation (shaving, etc.), the risk of local reactions, and the subjectivity of result interpretation. Serological tests (ASIS) avoid these drawbacks, but have limitations in terms of sensitivity and specificity; positive reactions can be observed in non-allergic dogs.
Ultimately, these allergy tests are only useful in determining which allergens to include in desensitization, and never for the diagnosis of canine atopic dermatitis.
Q2: How to choose allergens for an ASIT protocol?
The choice of allergens for an ASIT protocol should be based on the animal’s clinical history, in association with positive reactions to intradermal or serological tests. However, one should not rely solely on tests, as positive reactions can be observed in healthy dogs, reactions not necessarily reflecting clinical hypersensitivity. Careful interpretation of the clinical history and correlation with test results allows identification of the relevant allergens for establishing the therapeutic protocol. Provocation tests could be considered in some cases.
Q3: What are the advantages and disadvantages of rush immunotherapy (RIT)?
Rush immunotherapy (RIT) reduces induction duration compared to conventional immunotherapy. In dogs, RIT is well tolerated, major adverse effects are rare, and are mainly associated with pruritus. However, it requires inpatient clinical monitoring, and this type of approach carries greater safety risks, in addition to increased costs. RIT can be considered in dogs where the risk of anaphylaxis is low, saving time if tolerance is good. Optimization of protocols will require more in-depth evaluation to identify optimal safety parameters and avoid adverse reactions. Comparison between RIT and conventional immunotherapy is needed.
Q4: What are the new research avenues concerning canine ASIT?
Several research areas deserve to be explored: the use of modified allergenic preparations (allergoids, recombinant allergens, peptides), the development of new adjuvants to improve the efficacy and safety of treatment, the identification of biomarkers of therapeutic success, and the standardization of protocols. Exploration of innovative administration techniques, such as intralymphatic, epicutaneous immunotherapy, or oral administration is also promising. Research on canine ASIT is a constantly developing field, and new research avenues are always being explored. A standardization effort to improve the reliability of studies is essential.
Q5: Are there drug interactions to consider before performing skin tests?
Drug administration can influence IDT results. Certain drugs, such as antihistamines, glucocorticoids, ciclosporin, and tricyclic antidepressants can interfere with IDTs. A drug withdrawal period may be necessary before performing an IDT. Potential drug interactions must be considered before starting treatment. The effect of medications should be considered before performing the tests. A discussion with the veterinarian is essential to determine the potential discontinuation of some medications before performing the tests. Potential interactions with the medication used in the treatment of the disease must be taken into account.
Podcast
References
Hensel P, Santoro D, Favrot C, Hill P, Griffin C. Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification. BMC Veterinary Research 2015; 11:196. DOI: 10.1186/s12917-015-0515-5.
Mueller RS. Update on Allergen Immunotherapy. Vet Clin Small Anim 2019; 49: 1–7. DOI: 10.1016/j.cvsm.2018.08.001.
DeBoer DJ. The future of immunotherapy for canine atopic dermatitis: a review. Vet Dermatol 2017; 28: 25–66. DOI: 10.1111/vde.12416.
Pinto MSN, Gil SJRC, Ramió-Lluch L, et al. Challenging the norm: Epicutaneous immunotherapy for canine atopic dermatitis. Allergy 2023; 78: 255–257. DOI: 10.1111/all.15946.