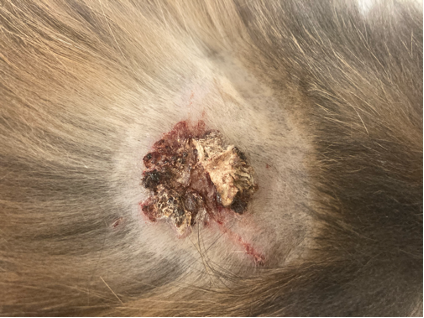
“Milky Way” is a 6-year-old FS domestic medium haired cat who presented for a refractory non-healing wound on the side of the face, dorsum, and axilla for 5 months prior to presentation.
Carine Laporte, VMD, DACVD
Dermatology for Animals, Salt Lake City, UT
DermaVetUSA
March 2024
History:
“Milky Way” developed firm, intensely pruritic, crusts on dorsal interscapular region, left zygomatic region, and left axilla approximately 5 months prior to presentation to the veterinary dermatologist. Dexamethasone injections had been used for 4 months with no improvement of the lesions or pruritus. There were no other pets in the house, and the owner was unaffected.
For years prior to the development of this lesion, “Milky Way” had a history suggestive of allergic dermatitis (steroid-responsive rodent ulcers on the lips and generalized pruritus). She had been variably treated with glucocorticoids and cyclosporine modified (Atopica®), but both had been discontinued due to adverse events. Subcutaneous allergen-specific immunotherapy based on serology had been initiated 2 years prior to presentation and was administered regularly.
Exam:
On physical examination, the skin and coat were unremarkable apart from an approximately 2cm diameter erosion to ulceration with very firm, adherent, mounded brown crust and two linear crusted erosions radiating from it, located on the dorsum near the interscapular region. Similar lesions were appreciated on the left lateral cheek and left axilla / proximomedial forelimb.
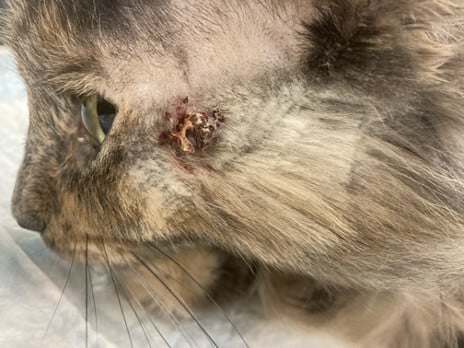
Images and case details courtesy of Dr. Liz Layne, DVM, DACVD (BluePearl Dermatology, Salt Lake City, UT)
Diagnostics:
Impression cytology showed numerous eosinophils and no microorganisms.
Punch biopsies of the skin from the dorsal and left cheek lesions were taken and submitted for histopathology. Histopathology revealed a central and regionally extensive area in which the epidermis was deeply ulcerated and within the ulcer bed was a thick, conical crust protruding from the skin surface and confluent with the necrotic coagulum of the ulcer bed. The crusts consisted of necrotic granulocytes, serum and amorphous necrotic material surrounding fragments of thick, hyalinized or coagulated collagen bundles. Additional thick, coagulated collagen bundles could be seen spanning from the dermis to merge with the coagulum of the ulcer bed and crust. Casts of keratin and hair shafts surrounded by necrotic follicles could be identified along the margins of the ulcer in one section. At the margins of the ulcers, the epidermis was markedly hyperplastic and spongiotic with multifocal pooling of mucus and mild to moderate parakeratotic hyperkeratosis. There was reactive fibroplasia, hemorrhage and a dense infiltrate of mixed inflammatory cells surrounding the ulcer beds in the dermis, and there was more dispersed but still marked inflammation concentrated around blood vessels and adnexa and extending diffusely throughout the dermis in all sections. Inflammatory cells included large numbers of eosinophils, lymphocytes, macrophages and mast cells with fewer plasma cells and neutrophils.
Assessment:
Clinical differential diagnoses included allergic dermatitis with concurrent reactive perforating collagenosis, Bowenoid in situ carcinoma / Bowen’s disease, and idiopathic ulcerative dermatosis.
Bowenoid in situ carcinoma are preneoplastic lesions that may be caused by solar exposure or papillomaviral infection. In the latter case, lesions are not restricted to sparsely haired or lightly pigmented regions like solar-induced lesions would be. Idiopathic ulcerative dermatosis involves ulcerative and crusting lesion(s) on the dorsal neck / dorsal interscapular region, which could fit with one of this patient’s lesions. However, the lesions on the check and in the axilla were not typical for this condition.
The histopathologic findings were most consistent with reactive perforating collagenosis.
Plan:
Betamethasone valerate 0.1% for application twice daily to the lesions and oral Vitamin C 100mg per day were initiated to reduce inflammation and promote reduction and regulation of collagen formation. Immunotherapy was continued.
Future avenues of therapy were recommended to the owner. This included further allergy work-up with a parasite treatment trial for potential parasite / flea bite hypersensitivity, and a 6-12-week strict, prescription, hypoallergenic diet trial with a hydrolyzed or novel protein source to evaluate for potential cutaneous adverse food reaction.
Discussion:
This is an interesting case of a rare disease in cats and humans called reactive perforating collagenosis.
Reactive perforating collagenosis is characterized by histopathologic findings of transepidermal elimination of altered collagen. Etiology and pathogenesis are poorly elucidated, though an association with aberrant wound healing is suspected in both humans and cats. This is suspected because the disease is more commonly reported in humans and cats with a history of traumatized skin (e.g., self-trauma associated with pruritus, surgical wounds, etc.). Correspondingly, this disease may be more common in cats with allergic dermatitis, possibly due to the predisposition of pruritic patients to self-traumatize; however, true association has not been established. In humans, superficial trauma, genetic predisposition, micro vasculopathy, and calcium deposits in the skin have been proposed as contributing factors.
Reactive perforating collagenosis in humans is found in two forms: an inherited form in childhood and an acquired form in adulthood. In the adult acquired form, it is more commonly found in patients with diabetes mellitus and end-stage renal disease. It has been proposed that a diabetic vasculopathy associated with scratching might cause dermal necrosis resulting from poor blood supply, with the necrotic material extruding through the epidermis. In patients with renal failure, dermal deposition of calcium may promote inflammation and connective tissue degradation, with subsequent collagen extrusion.
Most acquired cases in humans are self-limiting and spontaneously regress within 6-8 weeks. Though the disease tends to wax and wane / relapse throughout a patient’s lifetime, treatment is typically not needed. When prescribed, treatment is aimed at controlling pruritus with topical corticosteroids, emollients, and systemic antihistamines. Other therapies (keratolytics, topical and oral retinoids, phototherapy, liquid nitrogen, methotrexate, and allopurinol) have been reported without much efficacy, and it seems that response to therapy is patient-specific and can vary with severity of the lesions. When human patients were advised not to scratch and were treated with antihistamines, tranquillizers, hypnotics and phototherapy (UVB), the lesions disappeared within a few months. However, no randomized controlled treatment trials have been performed to establish standard of care.
In cats, only an adult (acquired) form has been reported. Like in humans, because this disease is so rare in cats, little is known about pathogenesis or treatment. Knowledge of the disease is based on a few small case reports. All reported cats have presented with exophytic adherent crusts and ulcerations that were usually (but not always) pruritic. Predispositions have not been established.
Differential diagnoses in cats vary based on the extent of the lesions but may include Bowenoid in situ carcinoma, idiopathic ulcerative dermatosis, cutaneous horns, eosinophilic granuloma complex, solar dermatitis / actinic keratosis, squamous cell carcinoma, and allergies (feline atopic skin syndrome, flea bite hypersensitivity, cutaneous adverse food reaction). First ruling out these more common diseases is an important step in the diagnosis of reactive perforating collagenosis.
Reported treatments in cats have included oral glucocorticoids up to 2 mg/kg/day (varying response), betamethasone 0.05-0.1% ointment, halofuginone, and oral ascorbic acid (Vitamin C) 100-250 mg / day. In one cat, betamethasone was effective when applied twice daily for 50 days, but the betamethasone-treated skin ultimately developed glucocorticoid-induced atrophy. Both the effectiveness for this condition and the adverse effects were attributed to betamethasone’s ability to inhibit collagens. Halofuginone, a type I collagen gene transcription inhibitor was also effective and did not cause cutaneous atrophy. Interestingly, one cat developed lesions at the sites of previous skin biopsy, supporting the concept of a role of aberrant wound healing.
Ultimately, when presented with patients who have lesions suggestive of reactive perforating collagenosis (adherent, thick crusts overlying ulcerations), first ruling out the much more common differential diagnoses listed above is important. This condition is so rare, it is likely that most veterinarians will not see it at all during their careers. Still, it is important to recognize because while the lesions in cats do not seem to consistently respond to typical allergy therapy like glucocorticoids, they can respond to other therapies (topical steroids, Vitamin C, halofuginone). Therefore, recognizing that this is a separate condition from allergic dermatitis is important. At the same time, because reactive perforating collagenosis may be associated with trauma to the skin, concurrently addressing any underlying allergic trigger may be necessary to resolve the lesions. This disease is a good example of a condition that can be diagnosed with high confidence on histopathology read by a pathologist experienced in dermatopathology, and biopsy should be considered in any patient with ulcerated and crusted lesions that do not respond to initial therapeutic attempts.
References:
- Albanese F et al. Feline perforating dermatitis resembling human reactive perforating collagenosis: clinicopathological findings and outcome in four cases. Veterinary Dermatology 2009;20:273-80.
- Scott DW et al. An usual perforating dermatitis in a Siamese cat. Veterinary Dermatology 1991;2:173–7.
- Beco L et al. Is feline acquired reactive perforating collagenosis a wound healing defect? Treatment with topical betamethasone and halofuginone appears beneficial. Letter to the Editor. Veterinary Dermatology 2010;21:434-6.